Stem Cell-derived Exosome Application
- Vascular Tissue Injury Repair
Overview Services Features FAQs
SC-Exo (Stem cell-derived exosomes) have an important role in vascular injury repair, and their mechanisms of action including anti-vascular inflammatory response, inhibition of oxidative stress, and anti-apoptosis have been shown in a wide range of research evidence. Creative Biolabs supports the exploration of the potential of SC-Exo isolation, profiling, and application related to vascular tissue injury repair.
Overview of Angiogenesis Mechanisms
Angiogenesis is a complex, tightly controlled process that is necessary for the growth, development, and restoration of injured tissue.
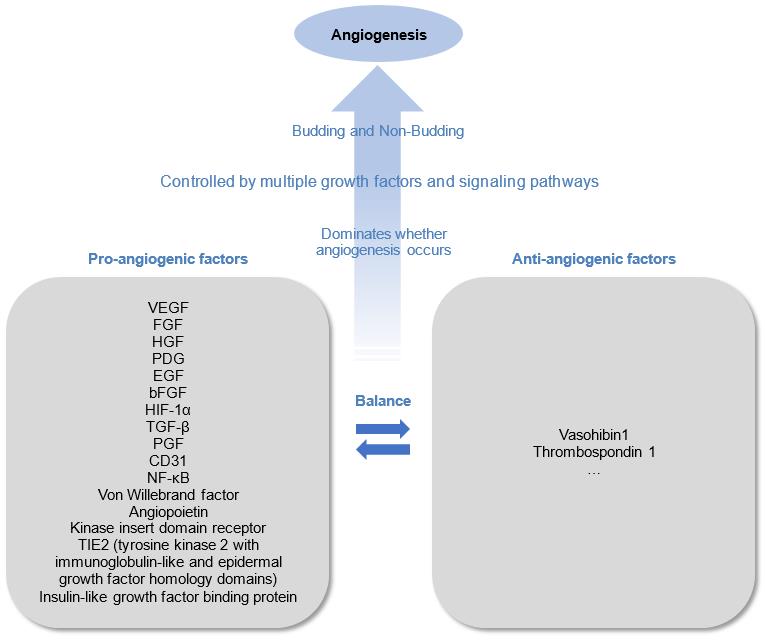 Fig.1 Angiogenesis Mechanisms.
Fig.1 Angiogenesis Mechanisms.
Vascular Tissue Injury and SC-Exo Exert Repairing Effects
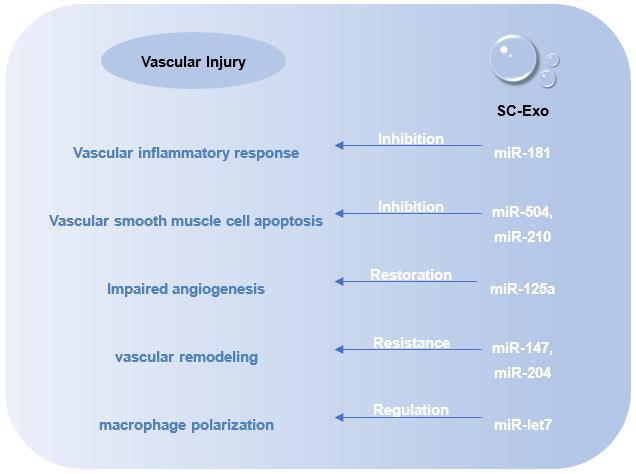 Fig.2 SC-Exo Repair Effects.
Fig.2 SC-Exo Repair Effects.
Vascular injury or dysfunction greatly affects the regeneration of tissues. SC-Exo has the biological function of reducing inflammatory response and suppressing immunity, and can specifically accumulate to the damaged area, which is an advantage in vascular injury repair. SC-Exo avoid immune rejection, low retention rate, low survival rate and tumorigenic risk of cell transplantation, and overcome these limitations.
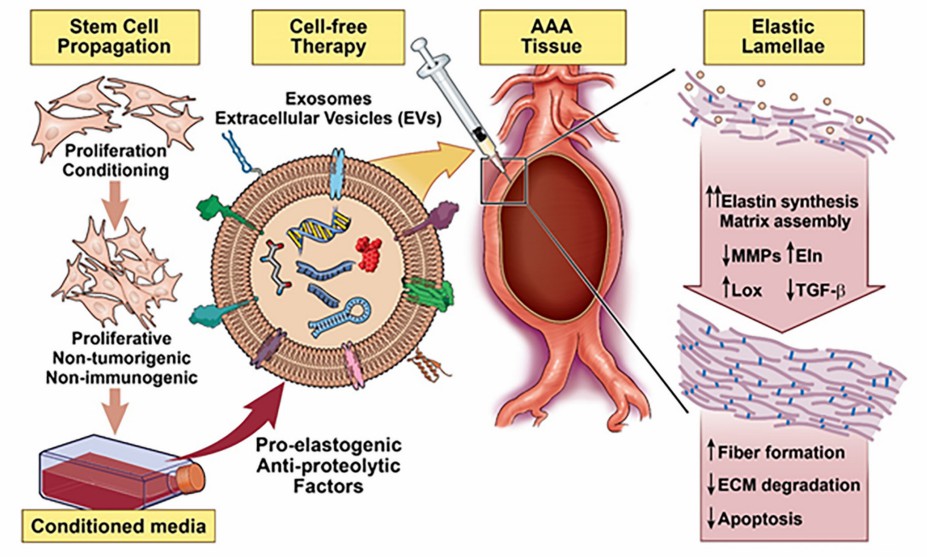 Fig.3 Vascular elastic matrix repair involves the delivery of SC-Exo.1, 3
Fig.3 Vascular elastic matrix repair involves the delivery of SC-Exo.1, 3
Different Sources of SC-Exo Promote Vascular Repair
|
SC-Exo Types
|
Applications
|
|
MSC-Exo (Mesenchymal SC-Exo)
|
Bone marrow MSC-Exo
|
Bone marrow MSC-EXO regulates miR-106a expression through lnc-H19 and modulate the expression of angiogenic factor Angpt1, thereby activating TIE2-NO signaling to promote osteogenesis and angiogenesis. Among them, a new bioactive molecule, lncRNA KLF3-AS1, was detected to promote angiogenesis in diabetic rats by downregulating mi R-383 and increasing the expression of its target VEGFA.
|
|
Umbilical MSC-Exo
|
Umbilical MSC-EXO promotes VEGFA and CD31 expression by transferring miR-1246 to target serine protease 23 to activate the Snail/α-SMA pathway.
|
|
Adipose MSC-Exo
|
RNA analysis of adipose MSC-EXO showed high expression of miR-21, miR-27b, miR-322 and let-7i, which contributed to improved angiogenesis and blood perfusion in hindlimb ischemic mice, an effect that was counteracted by exosome inhibitors.
|
|
iPSC-Exo
|
iPSC-EXO carrying pro-angiogenic factors promote tube formation and increase microvessel density in hindlimb ischemic mice by activating the PI3K/Akt pathway.
|
|
Endothelial progenitor cells-derived exosomes
|
High-throughput sequencing screening of miR-221-3p, which targets homeodomain interacting protein kinase 2, and miR-126, which targets sprouty-related EVH1 domain-containing protein 1, in exosomes increased VEGF, CD31 and other angiogenesis-related proteins.
|
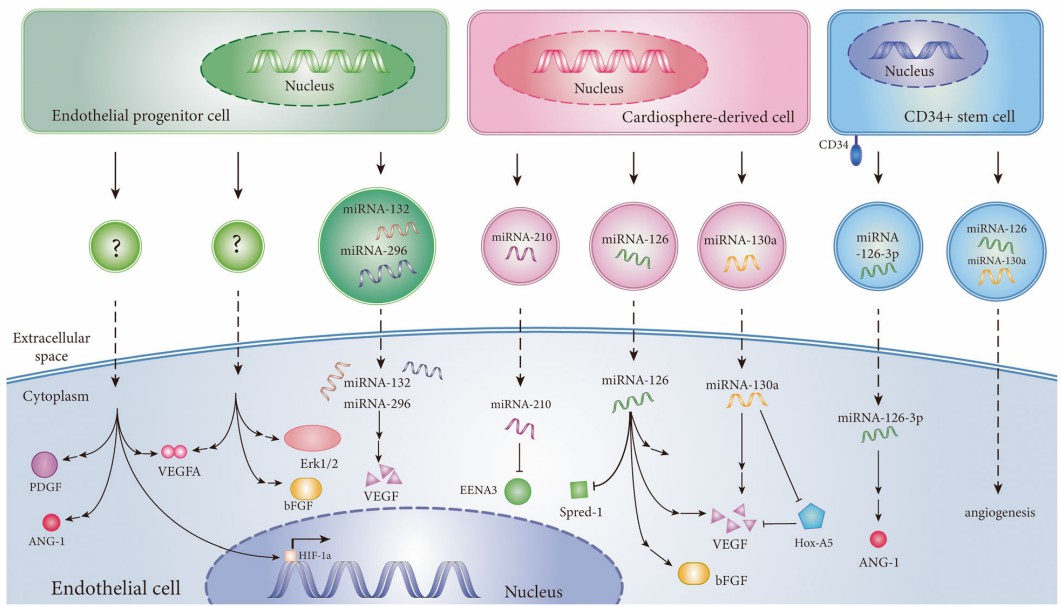 Fig.4 SC-Exo induces angiogenesis in ischemic diseases.2, 3
Fig.4 SC-Exo induces angiogenesis in ischemic diseases.2, 3
The potential of various SC-Exo in vascular tissue generation is very promising. Creative Biolabs helps to clarify the function and mechanism of SC-Exo in angiogenesis, which will help to enhance the theoretical value and practical significance of SC-Exo research. Please feel free to contact us.
FAQs
Q: How do stem cell-derived exosomes facilitate the repair of vascular tissue injuries at the molecular level?
A: Stem cell-derived exosomes can modulate inflammatory responses, promote angiogenesis, and enhance cellular proliferation and migration, thereby accelerating the repair processes in vascular tissues. The specific molecular pathways involved include the activation of growth factor signaling and the modulation of immune responses.
Q: What are the key differences in the therapeutic efficacy of exosomes derived from different types of stem cells (e.g., mesenchymal stem cells vs. induced pluripotent stem cells) in vascular repair?
A: Depending on where they originate from within the cell, exosomes can have varying degrees of therapeutic efficacy. Mesenchymal stem cell-derived exosomes are known for their immunomodulatory properties and ability to promote angiogenesis, while induced pluripotent stem cell-derived exosomes may exhibit enhanced regenerative capabilities due to their pluripotent nature. Comparative studies are essential to elucidate the specific mechanisms and outcomes associated with each type of stem cell-derived exosome in vascular repair.
Q: What are the potential challenges and limitations in the clinical translation of stem cell-derived exosomes for vascular tissue repair?
A: Several challenges exist, including the standardization of exosome isolation and characterization methods, ensuring consistent therapeutic efficacy, and addressing potential immunogenicity. Additionally, the scalability of exosome production and the establishment of optimal delivery methods to target vascular injuries are critical factors that need to be addressed before clinical application.
Q: How do the physicochemical properties of exosomes influence their biodistribution and therapeutic outcomes in vascular injury models?
A: The size, surface charge, and lipid composition of exosomes significantly affect their biodistribution and cellular uptake. Smaller exosomes may penetrate tissues more effectively, while surface modifications can enhance targeting to specific cell types within the vascular system. Understanding these properties is crucial for optimizing exosome formulations for improved therapeutic outcomes in vascular injury repair.
Q: What role do exosomal microRNAs play in the modulation of vascular repair processes, and how can they be harnessed for therapeutic purposes?
A: Exosomal microRNAs are key regulators of gene expression and can influence various cellular processes involved in vascular repair, such as apoptosis, inflammation, and angiogenesis. Identifying specific microRNAs that promote vascular regeneration allows for the development of targeted therapies, potentially using exosome-based delivery systems to enhance the therapeutic effects of these microRNAs in clinical settings.
Q: Are there any ongoing trials investigating the use of stem cell-derived exosomes in vascular tissue repair, and what are their preliminary findings?
A: Several trials are currently exploring the efficacy of stem cell-derived exosomes in various vascular injury contexts. Initial results point to encouraging results in terms of higher rates of healing and lower levels of inflammation. However, comprehensive data on long-term effects and safety profiles are still being gathered, highlighting the need for further research in this area.
References
-
Dahal, Shataakshi, et al. "Stem Cell Based Approaches to Modulate the Matrix Milieu in Vascular Disorders." Frontiers in Cardiovascular Medicine 9 (2022): 879977.
-
Bian, Xiaowei, et al. "Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases." Stem cell research & therapy 10.1 (2019): 1-18.
-
Under open access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Angiogenesis Mechanisms.
Fig.1 Angiogenesis Mechanisms.
 Fig.2 SC-Exo Repair Effects.
Fig.2 SC-Exo Repair Effects.
 Fig.3 Vascular elastic matrix repair involves the delivery of SC-Exo.1, 3
Fig.3 Vascular elastic matrix repair involves the delivery of SC-Exo.1, 3
 Fig.4 SC-Exo induces angiogenesis in ischemic diseases.2, 3
Fig.4 SC-Exo induces angiogenesis in ischemic diseases.2, 3









