Lentiviral Vector Optimization Service
Gene therapy has played an important role in a number of disease research, especially in various cancers. As a professional expert in lentiviral vectors, Creative Biolabs provides state-of-art technologies to meet any requirement of our customers. We have successfully completed a series of challenges in the past years. In particular, we have established an advanced lentiviral vector optimization platform which enables us to offer a series of high-quality service for the treatment of a collection of diseases.
Introduction of Lentiviral Vector in Gene Therapy
Gene Therapy
Gene therapy has been widely used in a number of disease treatment, such as tumors, as well as autoimmune diseases. In general, gene therapy is conducted by transferring therapeutic genes into specific cellular targets. These genes can recover the damages of gene variants, gene reprogramming, as well as defective cells, to improve the disease condition in animals or patients. For example, fusogenic membrane glycoprotein (GALV FMG) has shown its efficacy in cancer gene therapy. Pilot studies indicate that the lentivirus transfection system plays an important role in determining the efficacy of gene therapy.
Lentiviral Vector
In fact, gene transfer based on lentivirus has been proven its effectiveness, specificity, and stability for treating colon cancer, breast cancer, and diabetes. Meanwhile, three generations of the lentiviral vector have been identified based on the different packaged plasmids. The first generation consists of gag and pol sequences, several regulatory genes and accessory genes. Human immunodeficiency virus (HIV) related genes have been transferred into the former vector to generate the second-generation vector system. For the production of third-generation vector, genes, such as vif, vpr, vpu, and nef have been removed to enhance the safety of current vectors. In addition, recent studies have been focused on improving the functions of lentiviral vectors and a wide variety of methods have been established. Among them, glycoproteins, ligands, and promoters have become attractive targets for optimizing lentiviral vectors.
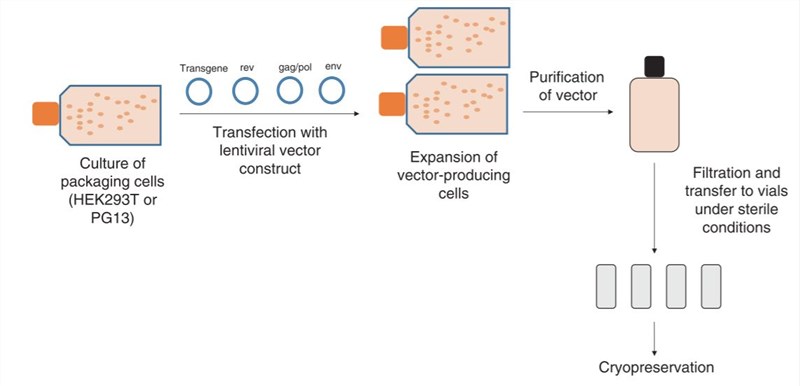 Figure 1. Manufacture of a lentiviral vector.1
Figure 1. Manufacture of a lentiviral vector.1
Service at Creative Biolabs
Gene therapy based on lentiviral vector has shown promising results in a range of disease therapy. Creative Biolabs now offers a large collection of lentiviral vector optimization services covering the entire lentiviral vector design, construction, safety assessment process, including but not limited to:
Glycoprotein Optimization of Lentiviral Vector
Recent studies have illustrated that glycoprotein represents great potential for treating a series of diseases, especially for cancer therapy. At Creative Biolabs, we provide pseudotyping services to expand the nature tropism and improve the stable expression of lentiviral vectors in a variety of cells. To date, we have developed many vectors targeting various cells, such as the central nervous cells, and lentiviral vectors have been pseudotyped using glycoproteins from the rabies virus.
Ligand-retargeted Lentiviral Vector Service
Recently, our expert team has developed a ligand-retargeted platform for designing novel lentiviral vectors to improve the targeting specificity in numerous cell types. We have discovered a method for cell-specific targeting of lentiviral vectors by using the interaction of receptor-ligand proteins. For example, erythropoietin protein has been fused to TVA or TVB receptors in our company. The data show that these ligand-receptor fusion proteins can trigger cell transduction to express specific Epo receptor.
Tissue-specific Promoter-Regulated Lentiviral Vectors Service
Scientists have theorized that the critical part of gene therapy against diseases requires suitable therapeutic vectors. Recent studies suggest that a number of lentiviral vectors, particularly for the tissue-specific lentiviral vector, should be perfect targets. Promoters have been generated and widely used for modifying lentiviral vectors to infect a variety of cells. For instance, we have designed two lentiviral vectors based on CD40L proximal promoter to treat X-linked hyper-immunoglobulin M syndrome (HIGM1) in animal models.
miRNA-regulated Lentiviral Vectors Service
The lentiviral vector has been considered as a powerful gene delivery system because of the high transduction in nondividing cells. MicroRNAs (miRNAs) are small non-coding RNAs and often expressed in a number of tissue or cells, which indicates that miRNA is a key target for regulating cellular gene function. More recent studies conducted by our labs have proved that several artificial sites of microRNA can regulate the transgene expression of lentiviral vectors. In this condition, we provide the optimization services of lentiviral vectors based on miRNA. Our standardized protocol and rigorous quality control system enable us to provide perfect solutions for your project.
Optimization of Bicistronic Lentiviral Vector Service
Bicistronic lentiviral vectors can deliver the stable expression of genes in disease therapy. This vector system is usually made up by ribosome entry site (IRES) elements, a furin cleavage site, and an amino acid spacer. It can regulate the expression level of T cell receptors (TCRs) in different types of lymphocytes. Currently, Creative Biolabs has developed a range of bicistronic lentiviral vector optimization services for our worldwide customers to identify proteins of interest by various detectable markers. In our assay, peptide tags and different 2A peptide linkers are widely used for increasing TCR expression.
The Creative Biolabs Advantage
- Depth of Expertise: Our team comprises virologists, molecular biologists, and bioinformaticians with direct experience in bringing LV-based therapies to the clinic. We understand both the molecular nuances and the regulatory hurdles.
- Proprietary Technology Suite: Access to a vast, curated inventory of optimized backbones, promoters, envelopes, and regulatory elements, many developed in-house and not available commercially.
- Focus on Challenging Applications: We excel where standard vectors fail: large or complex transgenes (e.g., dystrophin minigenes), difficult-to-transduce cells, and in vivo delivery schemes.
- Full Workflow Integration: Our service is not an isolated product. We offer seamless downstream integration with our viral vector manufacturing, cell line development, and analytical validation services, providing a single-vendor path from concept to studies.
- Unmatched Data Rigor: We deliver not just virus, but a comprehensive characterization package that strengthens your publication, grant application, or regulatory submission.
Result Delivery: Beyond the Viral Stock
Upon project completion, you receive:
- Finalized, sequence-verified plasmid DNA of the optimized transfer vector.
- High-titer, ready-to-use lentiviral preps (typically >1x10^8 TU/mL on permissive cells, with higher titers available).
- A detailed technical report including all QC data: physical/functional titers, transduction efficiency on target cells, expression kinetics, and copy number analysis.
- Standardized protocols for your specific vector's use and amplification.
Trusted by Global Innovators
"Partnering with Creative Biolabs was pivotal for our CAR-NK program. Our chimeric antigen receptor construct was large and unstable in standard backbones. Their team iteratively optimized the vector architecture, incorporating a proprietary promoter and RNA stability element, resulting in a 10-fold increase in functional titer and uniform, persistent CAR expression in primary NK cells. This enabled the critical preclinical proof-of-concept study for our IND."
– Director of Research, Biotech Startup, North America
"For our neuroscience research requiring precise in vivo targeting of dopaminergic neurons, their pseudotyping expertise was invaluable. They delivered a rabies-G pseudotyped vector with a neuron-specific promoter that showed exceptional specificity and retrograde tracing capability in our rodent models, significantly enhancing the impact of our published work in Nature Neuroscience."
– Principal Investigator, Top-Tier Academic Institute, Europe
Frequently Asked Questions
Q: How do you handle "difficult-to-package" genes?
A: Large genes (>4kb) or toxic proteins can reduce viral titers. We employ specific strategies such as using weaker promoters during packaging to reduce toxicity to producer cells or splitting the gene across dual-vector systems for larger cargoes.
Q: What is the difference between physical and functional titer?
A: Physical titer measures the amount of viral protein (p24) or RNA, while functional titer measures the actual number of infectious units capable of transducing cells. We prioritize functional titer as it is the biologically relevant metric for your experiments.
Q: What is your starting material requirement?
A: We can begin with a plasmid, a sequence file, or even a discussion of your gene of interest. Our team will guide you through the most efficient starting point.
Q: Can you optimize vectors for in vivo delivery?
A: Absolutely. This is a core specialty. We optimize for in vivo use through strategies like envelope pseudotyping for organ/tissue targeting (e.g., PHP.eB for CNS), promoter selection for cell-specificity, and incorporation of miRNA targets to detarget off-site cells (e.g., hepatocytes), thereby enhancing efficacy and safety.
Connect with Us Anytime!
As one of the top CROs in the field of lentiviral vector services, Creative Biolabs has focused on the development of gene therapies for years. We have accumulated extensive experience from the accomplishment projects and are very proud of our high-quality, omnidirectional lentiviral vector optimization services to meet any special need of your research. If you are interested in our services, please contact us or send us an inquiry.
Reference
- Michael, C.M.; et al. (2018). Clinical use of lentiviral vectors. Leukemia. 32: 1529-1541. 10.1038/s41375-018-0106-0 (Distributed under Open Access license CC BY 4.0, without modification.)
