ECLIA can detect analytes with picomolar to femtomolar sensitivity, often spanning more than six orders of magnitude in its dynamic range, enabling detection of both low and high concentration targets within a single assay.
At Creative Biolabs, we specialize in comprehensive electrochemiluminescence immunoassay (ECLIA) based kit development, empowering our clients with highly sensitive, robust, and customized diagnostic solutions. Leveraging our deep scientific expertise, we transform your biomarker concepts into market-ready assays, significantly accelerating your research and clinical applications.
How Does It Work?
ECLIA is a sophisticated analytical technique that marries the precision of electrochemistry with the sensitivity of chemiluminescence. At its core, ECLIA employs electrochemiluminescent labels, most commonly ruthenium derivatives, conjugated to antibodies or antigens. When an electrical potential is applied to an electrode, typically carbon or platinum, in the presence of a co-reactant like tripropylamine (TPA), these labeled molecules undergo a series of redox reactions. This generates an excited state that subsequently emits light. The emitted light, which is exactly proportional to the concentration of the target analyte, is then precisely measured by a photomultiplier tube. The use of magnetic beads coated with streptavidin allows for efficient separation and washing steps, minimizing background noise and ensuring high signal-to-noise ratios.
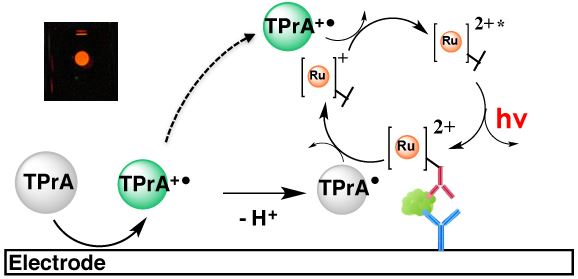 Fig.1 Mechanism of electrochemiluminescence. Distributed under CC BY-SA 4.0, from Wiki, without modification.
Fig.1 Mechanism of electrochemiluminescence. Distributed under CC BY-SA 4.0, from Wiki, without modification.
Advantages of Our ECLIA Platform
Exceptional Sensitivity and Dynamic Range
Reduced Matrix Effects
Unlike some optical detection methods, the electrochemical initiation of light emission in ECLIA significantly minimizes interference from complex biological matrices, leading to more consistent and reliable results across diverse sample types.
Lower Sample Volume Requirements
The high sensitivity of ECLIA often allows for accurate measurements with significantly smaller sample volumes, making it ideal for precious clinical specimens or high-throughput screening applications.
Enhanced Reaction Control
The ability to initiate and control the light-emitting reaction precisely through the application of an electrode potential offers superior reproducibility and control over assay kinetics.
Low Background Signal
The distinct nature of electrochemical excitation results in very low background optical signals compared to photoluminescence-based methods, contributing to higher signal-to-noise ratios.
Versatility and Automation
ECLIA platforms are highly amenable to automation, facilitating high-throughput processing and integration into complex laboratory workflows, thereby enhancing efficiency and reducing manual errors.
Our ECLIA Based Kit Development Services
At Creative Biolabs, we provide a meticulously designed, end-to-end ECLIA based kit development service tailored to your unique project requirements. From initial concept validation to comprehensive kit optimization and validation, our expert team collaborates closely with you at every stage. We focus on developing custom assays for diverse analytes, including proteins, peptides, small molecules, and nucleic acids, ensuring optimal performance characteristics such as sensitivity, specificity, linearity, and reproducibility. Our service encompasses everything from antibody selection and labeling strategies to solid-phase optimization, reagent formulation, and robust quality control, delivering a complete, ready-to-use kit that meets your precise specifications and regulatory needs.
Applications
Clinical Diagnostics
ECLIA is widely used for routine diagnostic testing, including the quantification of hormones (e.g., thyroid hormones, steroid hormones), tumor markers (e.g., PSA, AFP), cardiac markers, and infectious disease antigens and antibodies (e.g., HIV, Hepatitis B/C, SARS-CoV-2). Its high sensitivity is crucial for early disease detection and monitoring.
Biomarker Discovery and Validation
The technology is instrumental in identifying and validating novel biomarkers for various diseases, supporting drug discovery and development efforts by enabling sensitive detection of target analytes in complex biological matrices.
Therapeutic Drug Monitoring (TDM)
ECLIA provides accurate and rapid measurement of drug concentrations in patient samples (e.g., immunosuppressants, antibiotics), allowing for personalized dosing to optimize therapeutic efficacy and minimize adverse effects.
Food Safety and Environmental Testing
Beyond human health, ECLIA can be adapted for detecting contaminants, toxins, or allergens in food products and environmental samples, ensuring public safety and regulatory compliance.
Research and Preclinical Studies
In academic and pharmaceutical research, ECLIA facilitates high-throughput screening, protein interaction studies, cell signaling pathway analysis, and characterization of biologics, accelerating basic scientific understanding and drug candidate evaluation.
Published Data
1. A Novel Highly Sensitive and Throughput Electrochemiluminescent Immunoassay System
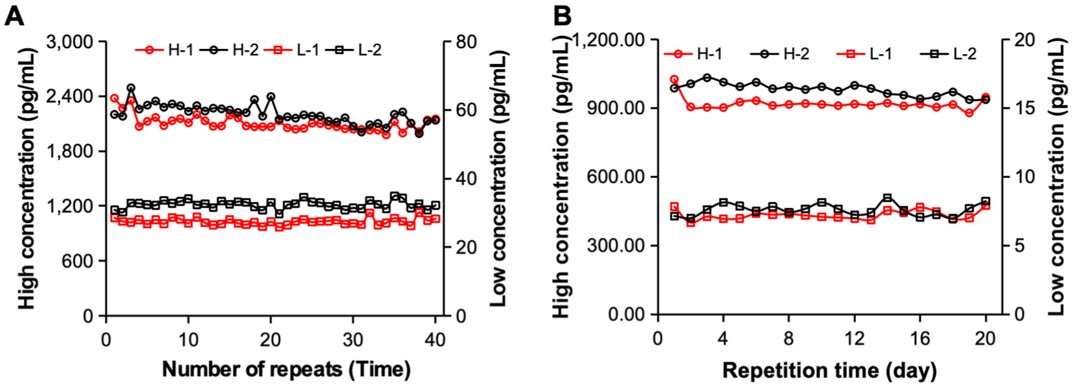 Fig.2 The detection precision of the ECLIA system.1,3
Fig.2 The detection precision of the ECLIA system.1,3
This study developed an electrochemiluminescence immunoassay (ECLIA) system to meet the demand for high-accuracy, fast clinical testing. By incorporating magnetic separation modules, introducing a photomultiplier tube (PMT), and optimizing system timing, the system enhanced detection accuracy, sensitivity, and throughput. The magnetic separation improved accuracy, the PMT boosted sensitivity, and the timing optimization maximized test output. Finally, this study evaluated the overall performance of the ECLIA system which demonstrated excellent performance in linearity, signal-to-noise ratio, detection limit, reproducibility, and accuracy. Overall, this optimized ECLIA system offers high-throughput, sensitive, and reliable detection, facilitating its potential clinical application.
2. A Novel Electrochemiluminescence Immunoassay for Measuring IgM-Free AIM
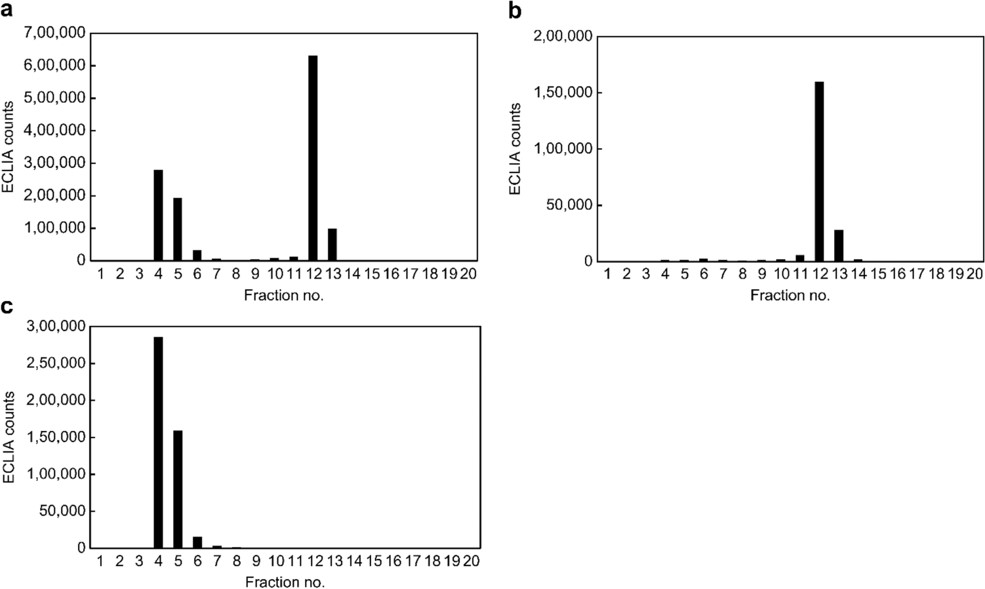 Fig.3 Specificity evaluation of the ECLIA for IgM-free AIM.2,3
Fig.3 Specificity evaluation of the ECLIA for IgM-free AIM.2,3
In this work, researchers developed a specific antibody targeting IgM-free apoptosis inhibitor of macrophage (AIM) and established a fully automated, high-throughput electrochemiluminescence immunoassay (ECLIA) to quantify IgM-free AIM. The method was applied to assess the role of IgM-free AIM in diagnosing NASH-associated hepatocellular carcinoma (NASH-HCC) and viral HCC. Using ECLIA, they measured IgM-free AIM levels in 212 serum samples from patients with HCC related to NASH, hepatitis B, and hepatitis C. Additionally, they developed an ECLIA for total AIM (IgM-free and IgM-bound) and used size-exclusion chromatography to explore AIM's forms in the blood. This new ECLIA approach could enhance clinical studies on AIM and support HCC diagnosis.
Service Highlights
- Custom Antibody Sourcing & Optimization: We leverage our extensive network and expertise to select or develop the most effective capture and detection antibodies, critical for assay specificity and sensitivity.
- Proprietary Labeling Chemistries: Our advanced labeling techniques ensure stable and highly efficient conjugation of ruthenium labels to your target biomolecules, maximizing signal output and assay performance.
- Rigorous Validation Protocols: Each kit undergoes comprehensive validation, assessing critical performance parameters like linearity, accuracy, precision (intra- and inter-assay), and robustness across diverse sample matrices, ensuring reliable results.
- Scalable Manufacturing Solutions: We design development processes with scalability in mind, facilitating a seamless transition from prototype to large-scale production, supporting your commercialization efforts.
- Expert Regulatory Guidance: Our team provides insights and documentation support to help navigate the complex regulatory landscape, aiding in the successful approval and market entry of your diagnostic kits.
Q&A
-
Q: How do you ensure the sensitivity and specificity of the developed ECLIA kits?
A: Our approach to ensuring superior sensitivity and specificity involves multiple rigorous steps. We meticulously screen and select antibody pairs for high binding affinity and minimal cross-reactivity. During assay development, we optimize reagent concentrations, incubation times, and washing steps. Furthermore, comprehensive validation studies, including spike-and-recovery experiments, linearity assessments, and cross-reactivity panels, are conducted to confirm the kit's performance against relevant biological matrices and potential interfering substances.
-
Q: Can you develop ECLIA kits for automated platforms?
A: Absolutely. We understand the significance of automation in modern diagnostic and research laboratories. Our development process inherently considers compatibility with various automated ECLIA platforms. We optimize assay protocols, reagent formulations, and kit components to ensure seamless integration, high-throughput capability, and consistent performance when utilized on automated systems, thereby enhancing laboratory efficiency and reducing manual errors.
-
Q: What level of support do you provide after the kit development is complete?
A: Our commitment to our clients extends beyond the initial development phase. We offer comprehensive post-development support, including troubleshooting assistance, technical guidance for assay implementation, and assistance with scaling up production. Furthermore, we can collaborate on stability studies and provide documentation to support your regulatory submissions, ensuring a smooth transition from development to commercialization or routine use.
-
Q: Can you help with validating the developed kit against clinical samples?
A: While our core service focuses on the development and in-house validation of the ECLIA kit, we can certainly collaborate with clients to establish protocols and provide guidance for validating the developed kit against clinical samples. We can assist in designing studies to assess clinical sensitivity, specificity, and correlation with established methods, or prepare the necessary documentation to facilitate your own clinical validation studies.
-
Q: What is the typical sample matrix compatibility for your developed ECLIA kits?
A: Our ECLIA kits are developed with broad sample matrix compatibility in mind, including human serum, plasma, urine, saliva, and cell culture supernatants. During the development phase, we rigorously assess and optimize the assay for interference effects from various matrices. This ensures robust performance and reliable results across diverse biological sample types relevant to your specific application, minimizing the need for extensive sample pretreatment.
The importance of electrochemiluminescence techniques detection for bio-related applications has been well established. ECLIA is heavily used commercially for many clinical lab applications. To assist customers for specific detection of disease-related proteins, Creative Biolabs provides a variety of ELISA based assay kits. If you are interested in our chemiluminescent immunoassay based kits development, please feel free to contact us for more details.
References
- Liu, Xiancheng, et al. "A Novel High-Throughput and Sensitive Electrochemiluminescence Immunoassay System." Bioengineering 11.9 (2024): 885.
- Shimizu, Tomo, et al. "Hepatocellular carcinoma diagnosis using a novel electrochemiluminescence immunoassay targeting serum IgM-free AIM." Clinical Journal of Gastroenterology 15.1 (2022): 41-51.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.