Achieves significantly lower limits of detection, crucial for early disease diagnosis and detection of low-abundance biomarkers.
Creative Biolabs offers cutting-edge time-resolved fluorescence immunoassay (TRFIA) based kits development for highly sensitive, robust, and quantitative diagnostic solutions. Leveraging our deep expertise in TRFIA, we help our clients overcome assay interference and achieve superior detection limits. Partner with us for high-quality, cost-effective, and fully optimized kits that accelerate your project's success.
How Does It Work?
TRFIA, a sophisticated immunoassay technology, dramatically enhances signal detection by exploiting the unique photophysical properties of lanthanide chelates, such as Europium, Samarium, or Terbium. Unlike conventional fluorophores, these specialized labels exhibit long fluorescence decay times (microseconds to milliseconds) after excitation by a pulsed light source, which is significantly longer than the nanosecond decay of autofluorescence from biological samples or plasticware. By introducing a delay between excitation and signal measurement, TRFIA effectively eliminates short-lived background noise, enabling the detection of specific, long-lived signals with extraordinary clarity and sensitivity.
This time-gated detection mechanism can be achieved through various high-resolution techniques (depending on the required sensitivity and time resolution), including:
- With fast-detection electronics (nanoseconds and slower)
- With Time Correlated Single Photon Counting, TCSPC (picoseconds and slower)
- With a streak camera (picoseconds and slower)
- With intensified CCD (ICCD) cameras (down to 200 picoseconds and slower)
- With optical gating (femtoseconds-nanoseconds)
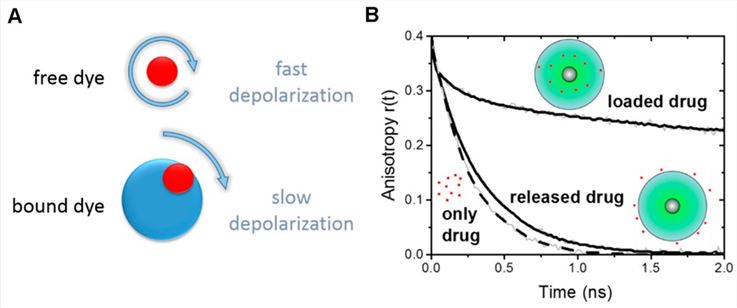 Fig.1 The detection precision of the ECLIA system.1, 4
Fig.1 The detection precision of the ECLIA system.1, 4
Advantages of Our TRFIA Platform
Exceptional Sensitivity
Superior Specificity
Time-resolved detection eliminates non-specific background fluorescence, leading to highly accurate results and fewer false positives.
Reduced Matrix Interference
Minimizes interference from endogenous biological compounds due to the time-gated measurement and large Stokes shift.
Wide Dynamic Range
Enables accurate quantification across a broad spectrum of analyte concentrations, simplifying sample analysis.
Enhanced Reagent Stability
Lanthanide labels offer remarkable photostability, contributing to longer kit shelf-life and consistent performance.
Easily Miniaturized
Compatible with miniaturized formats, making it suitable for high-throughput screening and automation.
Our TRFIA Based Kit Development Service
Creative Biolabs offers a comprehensive, customized TRFIA based kit development service tailored to your specific research and diagnostic needs. Our expert team takes a holistic approach, from initial concept to a fully validated and ready-to-deploy kit. We provide end-to-end support, including meticulous antigen and antibody selection, optimal lanthanide labeling strategies, rigorous assay optimization for sensitivity and specificity, and robust validation studies to ensure consistent performance. Whether you require a sandwich, competitive, or multiplex assay format, our service is designed to deliver highly accurate, reliable, and scalable TRFIA solutions that meet your project specifications and accelerate your progress from R&D to market.
Applications
Clinical Diagnostics
Enables early and accurate detection of diseases by identifying biomarkers present in low concentrations. This includes neonatal screening for metabolic disorders, hormone analysis for endocrine imbalances, and infectious disease diagnostics for pathogens like viruses and bacteria. Its precision is also critical for therapeutic drug monitoring, ensuring optimal dosing and preventing toxicity.
Drug Discovery and Development
Supports high-throughput screening (HTS) of drug candidates by providing rapid and sensitive detection of molecular interactions. It is invaluable for pharmacokinetic (PK) and pharmacodynamic (PD) studies, offering precise quantification of drug levels and their effects in biological systems. Additionally, TRFIA aids in the development and validation of new biomarkers for disease progression and therapy response.
Environmental Monitoring
Provides highly sensitive methods for detecting trace levels of pollutants, toxins, and contaminants in environmental samples, including water, soil, and air, contributing to public health and safety assessments.
Food Safety Testing
Used for the accurate detection of allergens, pathogens, pesticides, and other contaminants in food products, safeguarding consumer health and ensuring compliance with food safety regulations.
Published Data
1. Dual-Label Time-Resolved Fluorescence Immunoassay for Simultaneous Detection
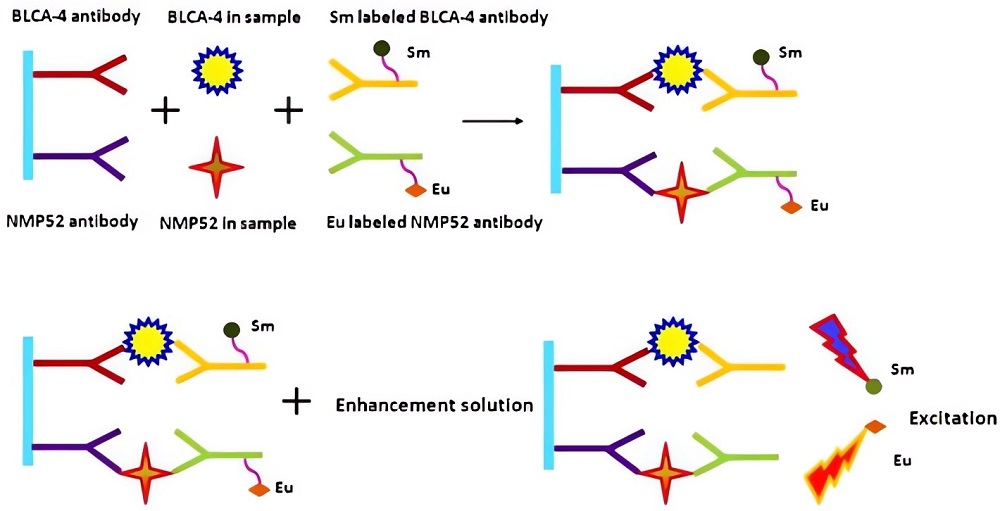 Fig.2 Schematic representation of the dual-label TRFIA for BLCA-4 and MNP52 detection.2,4
Fig.2 Schematic representation of the dual-label TRFIA for BLCA-4 and MNP52 detection.2,4
This study aimed to develop a dual-label time-resolved fluoroimmunoassay (TRFIA) capable of simultaneously detecting BLCA-4 and NMP52 within a single run. The assay employed a sandwich immunoassay format, in which anti-BLCA-4 and anti-NMP52 antibodies were immobilized on microtiter wells to capture BLCA-4 and NMP52 from the urine sample. These targets were then bound by europium (III) Sm3+ and samarium (III) Eu3+-labeled antibodies, followed by fluorescence measurement using time-resolved fluorometry. The assay's performance was evaluated using clinical urine samples and compared with commercial kits. The sensitivity of this method demonstrated 2 U/mL for BLCA-4 and 1 μg/mL for NMP52. The dual-label TRFIA demonstrates high sensitivity, specificity, and accuracy, having good prospects for clinical applications.
2. A Novel Time-Resolved Fluoroimmunoassay for Quantitative Detection
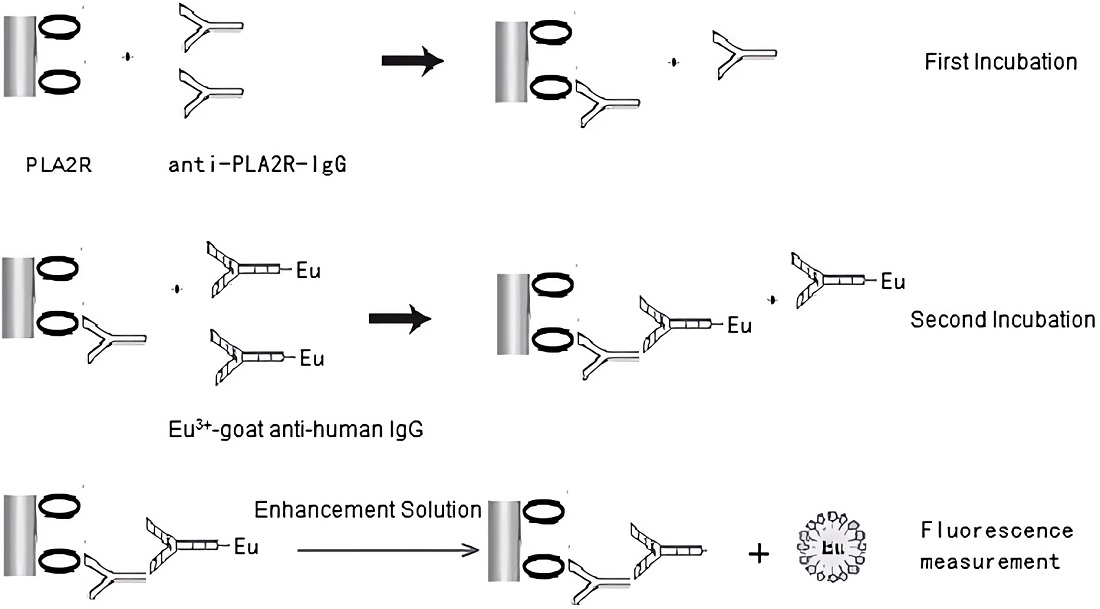 Fig.3 A schematic representation of the anti-PLA2R-IgG-TRFIA.3,4
Fig.3 A schematic representation of the anti-PLA2R-IgG-TRFIA.3,4
In this work, researchers established an ultrasensitive time-resolved fluoroimmunoassay (TRFIA) to measure anti-phospholipase A2 receptor (PLA2R) antibodies (anti-PLA2R-IgG) in serum for the membranous nephropathy differential diagnosis. Recombinant PLA2R (rPLA2R) was immobilized on 96-well plates for antigen capture, and a europium-chelate-conjugated goat anti-human IgG tracer was used for detection. After washing to separate bound/free components, fluorescence measurements quantified anti-PLA2R-IgG levels. A purified anti-PLA2R-IgG calibrator ensured consistent results. The assay had a detection limit of 0.03 mg/L and a linear range of 0.03–340 mg/L, with intra- and inter-assay coefficients of variation (CVs) of 3.8% and 6.2%, respectively. The method successfully detected anti-PLA2R-IgG concentrations in lupus nephropathy, IgA nephropathy, idiopathic membranous nephropathy patients and healthy volunteers. This quantification method enhances the diagnostic utility of serum anti-PLA2R-IgG for idiopathic membranous nephropathy.
Service Highlights
End-to-End Expertise
We manage the entire development lifecycle, from initial assay design and reagent selection to rigorous validation and comprehensive documentation. This allows you to leverage our specialized knowledge without needing to invest in complex in-house infrastructure or extensive training.
Customization at Its Core
Every project is unique. We provide fully customized solutions, tailoring assay formats, target analyte specifications, and detection strategies to precisely match your application requirements, ensuring optimal performance for your specific needs.
Uncompromising Quality Control
Our development process includes stringent quality control checks at each stage. We conduct thorough validation studies, including sensitivity, specificity, linearity, precision, and stability assessments, to guarantee the reliability and reproducibility of every kit.
Accelerated Development Timelines
By partnering with our experienced team, you can significantly reduce your project's research and development timeline, bringing your innovative diagnostic solutions to market faster and more efficiently.
Q&A
-
Q: Can your TRFIA kits be integrated into automated systems for high-throughput screening?
A: Absolutely. TRFIA is inherently well-suited for automation and high-throughput screening (HTS) applications. The stable fluorescence signal and broad dynamic range make it compatible with various robotic liquid handling systems and plate readers, allowing for efficient processing of large sample volumes and enabling rapid screening of compound libraries or patient samples.
-
Q: How do you promise the quality and consistency of the developed TRFIA kits?
A: Quality and consistency are paramount at Creative Biolabs. We employ a rigorous multi-stage quality control procedure that begins with strict incoming raw material examination. Throughout development, each batch of reagents is meticulously tested, and the final kits undergo comprehensive validation, including lot-to-lot consistency, stability studies, linearity, precision, and accuracy, adhering to stringent internal and, where applicable, regulatory guidelines.
-
Q: Are there any specific considerations for sample preparation when using TRFIA kits?
A: While TRFIA is highly robust against matrix interference, proper sample preparation remains crucial for optimal assay performance. For biological samples, this typically involves standard procedures like centrifugation to remove particulates. We provide detailed sample preparation guidelines with each kit, often recommending specific dilution buffers to ensure compatibility with the assay chemistry and maintain analyte integrity.
-
Q: Can your service accommodate the development of multiplex TRFIA assays?
A: Yes, our expertise extends to the development of multiplex TRFIA assays. By utilizing different lanthanide chelates with distinct emission spectra, we can simultaneously detect and quantify multiple analytes within a single sample well. This capability is highly valuable for gaining comprehensive insights from limited sample volumes, accelerating research, and improving diagnostic efficiency.
-
Q: How do you support clients through the regulatory approval process for diagnostic kits?
A: While Creative Biolabs is not a regulatory body, we provide extensive support to aid clients in navigating the regulatory approval process for their diagnostic kits. This includes supplying comprehensive documentation, detailed data packages from our validation studies, and expert scientific input. We ensure that our development processes and data collection methods are designed to meet the rigorous standards often required by regulatory agencies globally.
Creative Biolabs is a world leader in the development of ELISA based kits and offers a wide range of assay kits for specific detection of disease-related proteins. If you are interested in our ELISA kits development, please feel free to contact us for more details.
References
- Boreham, Alexander, et al. "Time-resolved fluorescence spectroscopy and fluorescence lifetime imaging microscopy for characterization of dendritic polymer nanoparticles and applications in nanomedicine." Molecules 22.1 (2016): 17.
- Liu, X., et al. "Development of a Dual-Label Time-Resolved Fluorescence Immunoassay (TRFIA) for Screening of Bladder Cancer based on Simultaneous Detection of BLCA-4 and NMP52 in Urine." J Bioprocess Biotech 7.303 (2017): 2.
- Huang, Biao, et al. "A novel time-resolved fluoroimmunoassay for the quantitative detection of antibodies against the phospholipase A2 receptor." Scientific reports 7.1 (2017): 46096.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.