Immuno-PCR achieves detection limits orders of magnitude lower than conventional ELISA, reaching attomolar to zeptomolar concentrations, critical for early disease detection and low-abundance biomarker analysis.
Immuno-PCR revolutionizes biomolecule detection by integrating PCR's exponential amplification with ELISA's specificity and versatility. As a leading innovator, Creative Biolabs provides comprehensive immuno-PCR based kit development services, enabling ultra-sensitive detection of low-abundance analytes crucial for clinical diagnostics, biomarker discovery, and environmental monitoring. We also provide other ELISA kits for the specific detection of disease-related proteins.
How Does It Work?
Immuno-PCR functions as a highly sensitive antigen detection system that leverages the robust amplification power of polymerase chain reaction. At its core, the technique involves an antigen-antibody complex, similar to traditional ELISA, where a target antigen is captured by a specific antibody immobilized on a solid surface. What distinguishes immuno-PCR is the subsequent step: a detection antibody, also specific to the antigen, is covalently linked to a unique DNA reporter molecule. Once this immunocomplex is formed and unbound components are washed away, the attached DNA fragment serves as the template for real-time PCR amplification. This process transforms a single antigen detection event into billions of DNA copies, which are then precisely quantified. This inherent mechanism overcomes the sensitivity limitations of conventional immunoassays like ELISA, which, despite their versatility for protein detection, often fall short when dealing with extremely low-abundance analytes in complex biological matrices.
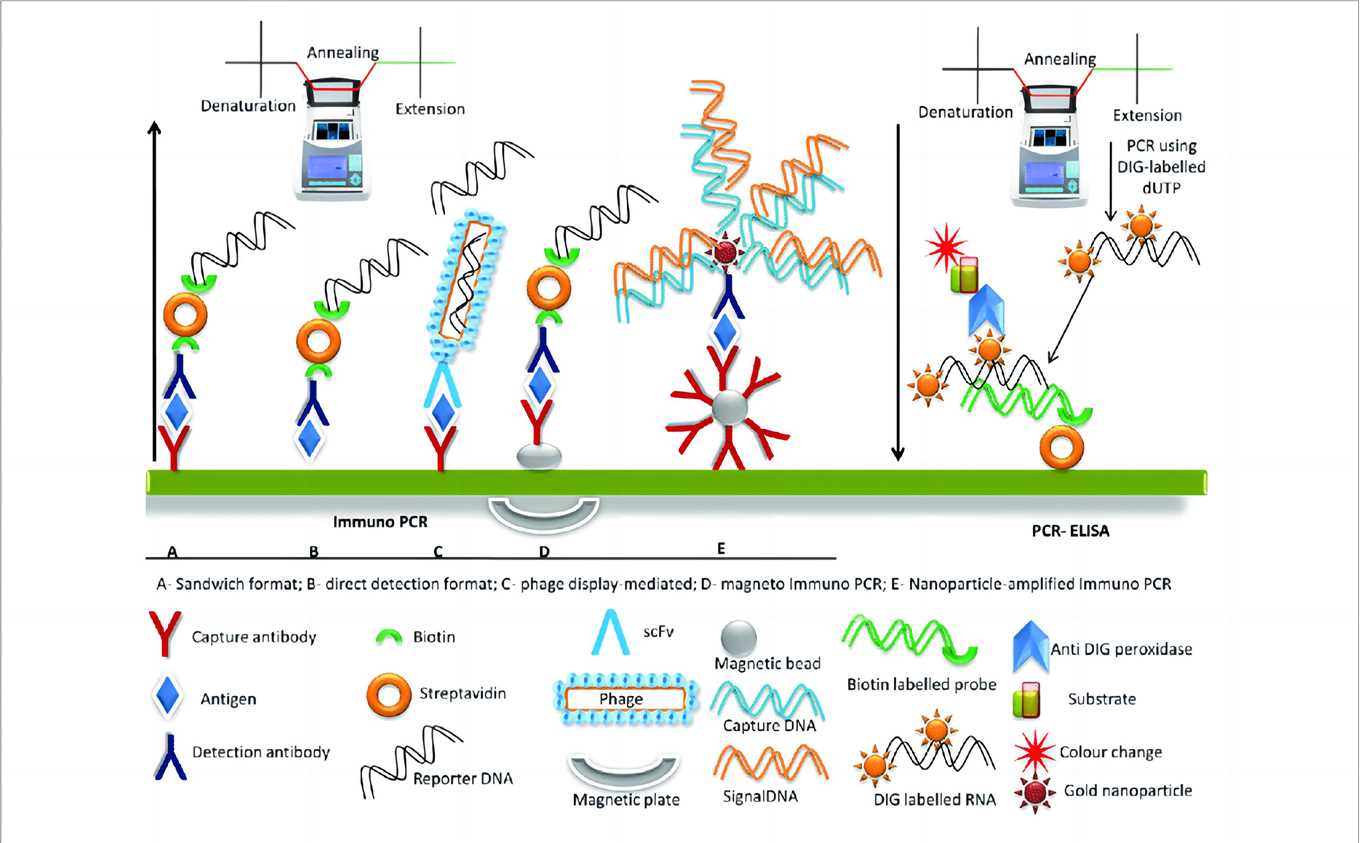 Fig.1 A schematic representation of PCR-ELISA and immuno-PCR1,4
Fig.1 A schematic representation of PCR-ELISA and immuno-PCR1,4
Advantages of Our Immuno-PCR Platform
Ultra-High Sensitivity
Enhanced Specificity
Combining antibody-antigen recognition with highly specific PCR primers dramatically reduces non-specific signals, leading to exceptionally accurate results.
Quantitative Precision
Real-time PCR allows for precise quantification of the target analyte across an extensive dynamic range, providing reliable data for research and diagnostics.
Reduced Sample Volume
The profound sensitivity of immuno-PCR often permits accurate analysis with significantly smaller sample quantities, invaluable in clinical settings with limited sample availability.
Broad Applicability
Its versatility extends across diverse sample types and analytes, including proteins, haptens, pathogens, and even cellular components, making it adaptable for various research and diagnostic needs.
Our Immuno-PCR Based Kit Development Services
Creative Biolabs specializes in developing customized immuno-PCR based kits engineered to meet your various detection demands. Our comprehensive service spans the entire development lifecycle, from initial concept and feasibility assessment to rigorous validation and optimization. We meticulously select and optimize antibody pairs, design efficient DNA reporter conjugation strategies, and develop highly specific PCR primers. Our expertise ensures optimal assay conditions, robust performance, and seamless integration into your workflow. Whether you need a highly sensitive assay for a novel biomarker or an improved diagnostic tool for an existing target, our dedicated team delivers tailored, high-quality, and cost-effective solutions that push the boundaries of detection.
Applications
Early Disease Diagnostics
Immuno-PCR enables the detection of disease biomarkers at extremely low concentrations, often long before symptoms manifest or conventional methods can provide a signal. This is crucial for early cancer screening, identifying infectious agents in initial asymptomatic stages, and detecting autoimmune disease onset. For instance, detecting trace amounts of tumor markers significantly improves prognostic outcomes.
Biomarker Discovery and Validation
Many promising biomarkers are present at very low concentrations in biological samples, making their discovery and validation challenging for traditional immunoassays. Immuno-PCR's ultra-sensitivity allows researchers to accurately identify and quantify these low-abundance markers, accelerating the discovery of novel diagnostic and prognostic indicators.
Forensic Medicine
The high sensitivity of immuno-PCR is particularly beneficial in forensic investigations where biological samples are often degraded or available in extremely limited quantities. It can be employed for the highly sensitive detection of specific human proteins, pathogens, or other biological markers in trace evidence, aiding in crime scene analysis and identification.
Food Safety and Environmental Monitoring
The ability to detect minute quantities of contaminants is vital for public health and environmental protection. Immuno-PCR can be utilized for the ultra-sensitive detection of foodborne pathogens, allergens, toxins, and environmental pollutants in complex matrices, ensuring compliance with stringent safety regulations.
Drug Discovery and Development
In pharmaceutical research, precise quantification of drug targets, therapeutic proteins, or specific immune responses is essential. Immuno-PCR provides the necessary sensitivity and specificity to monitor drug efficacy, study pharmacokinetics, and evaluate therapeutic protein levels, even at very low physiological concentrations, contributing to more efficient drug development processes.
Published Data
1. Ultrasensitive Immuno-PCR for Crustacean Tropomyosin Detection
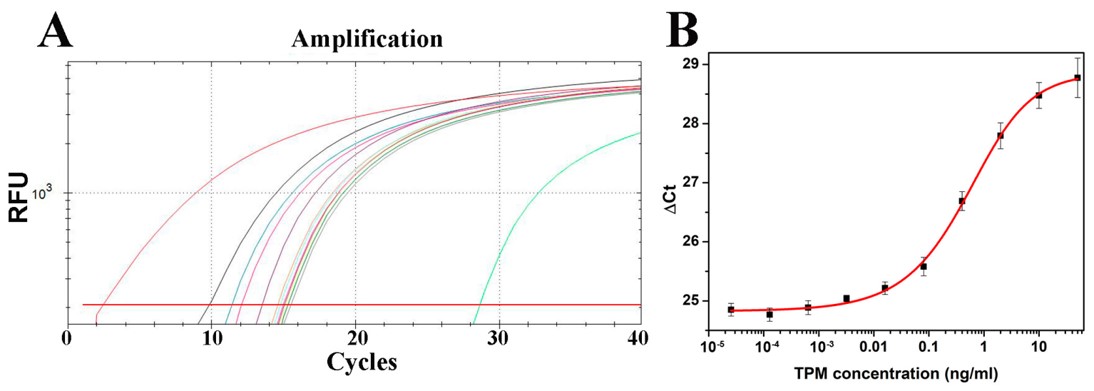 Fig.2 Crustacean TPM quantification results detected by immuno-PCR.2,4
Fig.2 Crustacean TPM quantification results detected by immuno-PCR.2,4
This study presented an ultrasensitive immuno-PCR method for quantifying crustacean tropomyosin (TPM), a major food allergen. The method combined sandwich ELISA and real-time PCR (rtPCR) for the amplification and detection of marker DNAs. A monoclonal anti-TPM antibody served as the capture antibody, while a polyclonal rabbit anti-shrimp tropomyosin antibody was used for detection, with natural shrimp TPM as the standard. A double-stranded amino-DNA was linked to an anti-rabbit secondary antibody and subsequently amplified through rtPCR. The immuno-PCR method offered 20-fold higher sensitivity than traditional ELISA, with a LOQ of 19.8 pg/mL. This assay was highly specific and precise across a broad concentration range. As the first immuno-PCR assay for food allergens, it provides a powerful tool for detecting trace amounts of TPM and could be adapted for the ultrasensitive detection of other food allergens currently quantified by ELISA.
2. Covalent and Cleavable Antibody-DNA Conjugates for Sensitive Immuno-PCR
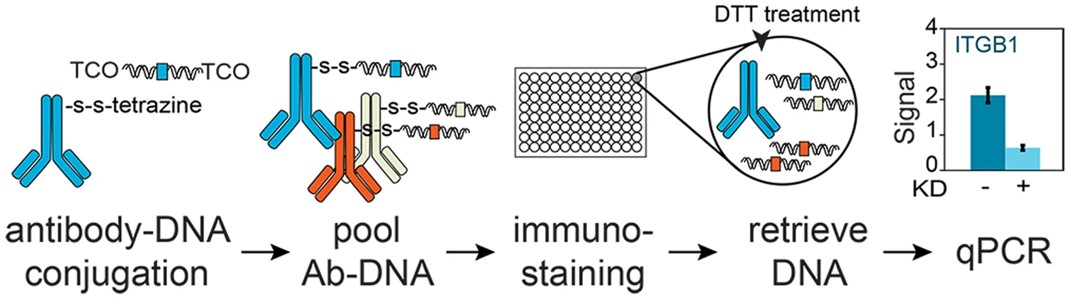 Fig.3 Overview of Immuno-PCR method using antibody-dsDNA conjugates.3,4
Fig.3 Overview of Immuno-PCR method using antibody-dsDNA conjugates.3,4
In this study, researchers developed a strategy for conjugating antibodies with double-stranded DNA (dsDNA) for sensitive immuno-PCR. The approach included a simple antibody functionalization step and a two-step dsDNA functionalization, followed by conjugation using tetrazine and trans-cyclooctene (TCO). A dithiothreitol (DTT)-cleavable linker enabled the release of dsDNA after immuno-staining for sensitive detection via qPCR. Different dsDNA sequences could be conjugated to antibodies, enabling multiplexed immuno-PCR. The strategy's throughput could be increased through parallel, miniaturized, or automated reactions. This chemically cleavable antibody-DNA conjugates will enable the development of multiplexed immuno-PCR and immuno-sequencing techniques.
Service Highlights
- Customized Solutions: Our services are meticulously tailored to your specific analyte, sample matrix, and desired sensitivity, ensuring the developed kit precisely meets your unique project requirements.
- Advanced Conjugation Chemistry: Leveraging proprietary and optimized methods for DNA reporter conjugation to detection antibodies, we ensure robust, stable, and highly efficient DNA loading, maximizing assay performance.
- Rigorous Validation: Every developed kit undergoes extensive validation, including detailed assessment of sensitivity, specificity, linearity, reproducibility, and robustness across various sample types, guaranteeing reliable and consistent results.
- Scalability for Production: We design our kits with future manufacturing in mind, optimizing protocols and components for potential large-scale production, facilitating a smooth transition from development to commercialization.
Q&A
-
Q: Can immuno-PCR be used for any protein, or is it limited to specific types?
A: Immuno-PCR is remarkably versatile, capable of detecting a wide range of proteins, haptens, pathogens, and even whole cells. The method hinges on specific antibody-antigen recognition. As long as specific, high-affinity antibodies can be developed or acquired for your target molecule, immuno-PCR can be adapted for detection, making it suitable for both abundant and extremely low-abundance analytes in diverse sample matrices.
-
Q: Is your immuno-PCR kit development service truly customized, or do you offer pre-designed kits?
A: Our service is exclusively focused on custom development. While we leverage our extensive expertise and proprietary methods, every immuno-PCR kit is meticulously designed and optimized from scratch to meet your exact specifications. We do not provide generic, pre-designed kits; instead, we partner closely with clients to create bespoke solutions that precisely address their unique research or diagnostic challenges.
-
Q: What is the minimum sample volume typically needed for immuno-PCR?
A: Immuno-PCR's exceptional sensitivity often translates to very small sample volume requirements. The precise minimum volume depends on the target analyte's concentration and your desired detection limit. However, immuno-PCR generally achieves reliable detection with significantly less sample than traditional ELISA, making it ideal for precious or limited samples often encountered in clinical research, pediatric diagnostics, or specialized research models.
-
Q: What quality control measures are in place during kit development?
A: Quality control is integrated throughout our development process, from raw material qualification to final validation. We conduct comprehensive checks for sensitivity (LoD), specificity, linearity, dynamic range, reproducibility, and robustness. All data is meticulously documented, ensuring high performance standards.
-
Q: How do you ensure the specificity of an immuno-PCR assay?
A: Specificity is multi-layered. We select highly specific antibody pairs for distinct epitopes and design PCR primers unique to the DNA reporter sequence. Rigorous validation, including testing against related analytes and matrix components, confirms the assay's precision.
Creative Biolabs is a world leader in the development of ELISA based kits and now offers Immuno-PCR based kits for specific detection of disease-related proteins. If you are interested in our ELISA kits development, please feel free to contact us for more details.
References
- Malik, Yashpal Singh, et al. "Advances in diagnostic approaches for viral etiologies of diarrhea: from the lab to the field." Frontiers in microbiology 10 (2019): 1957.
- Radomirović, Mirjana, et al. "Ultrasensitive Quantification of Crustacean Tropomyosin by Immuno-PCR." International Journal of Molecular Sciences 24.20 (2023): 15410.
- van Buggenum, Jessie AGL, et al. "A covalent and cleavable antibody-DNA conjugation strategy for sensitive protein detection via immuno-PCR." Scientific reports 6.1 (2016): 22675.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.

