Biophysical Characterization
Correct configuration of a product's higher-order structure (HOS), such as secondary and tertiary structure, aggregation and oligomerization, is critical to ensure the correct functionality and stability of a compound. As a service provider of preclinical drug discovery, Creative Biolabs provides several tools to help global customers investigate drug candidates’ higher-order structure.
During preclinical drug discovery, it is of great importance to analyze and characterize the higher-order structure (HOS) or conformation of protein-based drugs. To achieve this goal, Creative Biolabs has successfully established an advanced technology platform to help researchers investigate the correct functionality and stability of a compound. The techniques we provide for biophysical characterization including Dynamic Light Scattering (DLS), X-Ray Crystallography, Nuclear Magnetic Resonance (NMR) Spectrometry, Cryo-Electron Microscopy and Mass Spectrometry (MS).
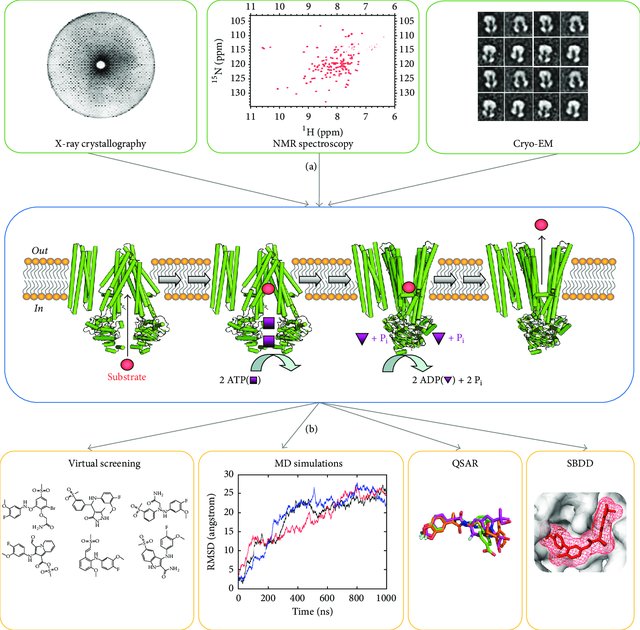 Fig.1 Experimental workflow from biophysical characterization of ABC proteins in multiple conformational states to in silico discovery and design of small molecule modulators.1
Fig.1 Experimental workflow from biophysical characterization of ABC proteins in multiple conformational states to in silico discovery and design of small molecule modulators.1
Dynamic Light Scattering (DLS)
DLS is a physical technique, which is capable of determining the particle size of very small particles and polymers in solution, even at low sample concentrations. The sample is illuminated by a laser beam and the temporal fluctuations are analyzed at a known scattering angle θ by means of the intensity or photon autocorrelation function. Elaborated multi-angle instruments can determine the full particle size distribution. In addition to measuring small molecules, DLS can also be used to probe the behavior of complex fluids, such as concentrated polymer solutions.
X-ray crystallography is a useful technique for exploring the atomic and molecular structure of a crystal, in which the crystalline atoms produce a bunch of X-rays to diffract a number of particular orientations. A crystallographer enables us to generate a three-dimensional (3D) drawing of the density of electrons within the crystal, by determining the angles and intensities of these diffracted beams. Based on our comprehensive X-ray crystallography platform, scientists from Creative Biolabs can offer tailored X-ray crystallography services to meet your demands for structure determination.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a cogent, lossless technique that utilizes the magnetic performance of certain atomic nuclei. NMR spectroscopy enables one to offer specified messages about the dynamics, reaction state, structure, and the chemical state of molecules. It can give access to particular information on the electronic structure of a molecule. This technique has been used to analyze the intact structure of complex molecules, quantificationally analyze the complex mixtures, and mild determinate the reaction rates of chemical systems. As an undisputed global leader in drug discovery, Creative Biolabs offers customized nuclear magnetic resonance (NMR) spectroscopy service to meet all your demands.
Cryo-electron microscopy, also referred to cryo-EM or electron cryomicroscopy, is a form of transmission electron microscopy (TEM). Usually, the samples are researched at cryogenic temperatures. Unlike X-ray crystallography, which needs to crystallize the specimen, cryo-EM enables the observation of specimens in their natural state. Cryo-EM density maps are commonly combined with NMR spectroscopy and X-ray crystallography to accomplish atomic-resolution patterns of intricate and actional molecular assemblies.
Mass spectrometry (MS) is a sensitive analytical technique that ionizes chemical species and detects, identifies and quantitates molecules based on their mass and charge (m/z) ratio. Creative Biolabs provides mass spectrometry service to support your drug discovery process. The MS platform can be used in many different fields and is applied to pure samples as well as complex mixtures.
Differential scanning fluorimetry (DSF) has successfully been used to screen protein libraries utilizing protein samples purified from 2 mL expression cultures and is useful for identifying appropriate conditions for protein and protein-ligand crystallization. Recently, instrument development for miniaturisation (nanoDSF) makes it a valuable tool to rapidly and reliably determine melting points of native proteins which allows measuring 48 samples in parallel in 10 μL volumes with < 10 μg of protein per determination.
Microscale Thermophoresis (MST)
MicroScale Thermophoresis technology (MST) is a powerful technique that allows for the fast, precise, cost-efficient, and quality-controlled characterization of molecular interactions with very low sample consumption, no need for sample immobilization, and a free choice of buffers or bioliquids. Most importantly, it could help researchers to determine the important parameters of molecular interactions, such as binding affinity (from pico-molar o milli-molar), binding stoichiometry, and interaction thermodynamics.
Multi Angle / Dynamic Light Scattering (MALS)
Dynamic Light Scattering (MALS) is a widely used technique in preclinical drug discovery to help researchers measure the size distributions of nano- and micro-particles dispersed in a liquid. Compared to single-angle DLS, MALS provides more robust, reproducible and accurate Particle Size Distributions (PSDs).
Quartz Crystal Microbalance (QCM)
The quartz crystal microbalance, comprised of a thin vibrating quartz wafer sandwiched between two metal excitation electrodes, has been used to determine interfacial mass changes through the mass dependence of the QCM resonant frequency. Currently, QCM has been widely used to detect various nanoscale target analytes in liquid and gas environments due to advantages including good surface selectivity, simplicity of operation, real-time output, label-free analysis, and so on.
Intrinsic tryptophan fluorescence (ITF)
Tryptophan fluorescence is the dominant source of intrinsic protein fluorescence. It has been used to determine the kinetics of protein-lipid and protein-protein interactions, the depth of membrane insertion of proteins, and the topology of peptide-membrane complexes.
Fluorescence polarization anisotropy (FPA)
Fluorescence polarization anisotropy (FPA) is a versatile solution-based technique that has been widely used to study molecular interactions, enzymatic activity, and nucleic acid hybridization. Traditionally, FPA is used to obtaining binding isotherms under carefully controlled settings to the study of small molecule-protein, antigen-antibody, and hormone-receptor binding in miniaturized automated settings. Currently, FP is adopted in high throughput screening to facilitate the drug discovery process, with its use being extended from direct interaction studies to complex enzymatic assays.
Analytical Ultracentrifugation (AUC)
SV-AUC is an analytical ultracentrifugation method that measures the sedimentation rate at which molecules move in response to the centrifugal force generated in a centrifuge. The sedimentation velocity depends on the instrument settings (angular velocity), the molecule properties (e.g., mass, density, and shape), and the carrier solution (density, viscosity). It has been widely used by the pharmaceutical industry to quantitatively characterize antibody aggregates, in particular, soluble antibody aggregates with diameters less than 100 nm, i.e. nanometer particles.
BLI technology refers to an optical analytic approach analyzing the interference pattern of white light reverberated from two surfaces: an inner reference layer, and a layer of settled protein on the biosensor tip. A variation in the interference pattern is induced by any altering in the number of molecules combined with the biosensor tip. In this case, interactions can be gauged in real time, offering the capacity to supervise binding specificity, the ratio of association and dissociation, concentration, with fidelity and accuracy.
Surface Plasmon Resonance (SPR)
SPR biosensors have turned into a standard technique within the biotechnology and pharmaceutical industries. It enables to identify the interactions of almost any molecular system. The two pivotal advantages of SPR technology are real-time analysis and no labeling necessities. The short of labeling decreases the time demanded to prepare samples for determination and eliminates the concern that a tag may influence the reaction. Real-time monitoring allows it feasible to extract particular information about binding events, containing the association and dissociation reaction kinetics.
A thermal shift assay is also known as differential scanning calorimetry refers to quantifying the variation in thermal denaturation temperature of protein in different surroundings. A variety of conditions enable to be determined, such as drugs, drug leads, mutations, salts, pH, and oxidation/reduction. Through this assay, protein thermal stability, appropriate buffer conditions, and protein-ligand interactions could be determined. Besides, thermal shift assay is able to be applied to hit identification and target engagement of drug candidates.
Isothermal Titration Calorimetry (ITC)
ITC is a physical method used to measure the heat exchange connected with molecular interactions at permanent temperature, mostly as an approach to detecting thermodynamic parameters connected with complex formation. Recently, ITC is applied to measuring the binding affinity of ligands for proteins and representatively used as a hit screening technique in high throughput screening.
Differential Scanning Calorimetry (DSC)
Creative Biolabs provides precise measurements of heats by DSC and gives sufficient information to support successful purity determinations. The DSC is the simplest analytical method for getting information about the purity and the crystal form of the sample under investigation. Temperature provided can range from -120°C to 725°C and the temperature is measured with repeatability of ±0.1°C.
Highlight Features
- Comprehensive biophysical characterization platform with professional scientists
- High reproducibility, short turnaround time, and relatively low price
- Flexible assay design that can be readily customized to satisfy your need
- Reliable, repeatable results deliver
Creative Biolabs is a global contractor for preclinical research in pharmaceuticals with a very professional scientific team. Our experienced scientists are dedicated to offering remarkable biophysical characterization services to help you complete your research. For more detailed information, please feel free to contact us or directly send us an inquiry.
Reference
- Molinski, Steven V., et al. "Biophysical Approaches Facilitate Computational Drug Discovery for ATP‐Binding Cassette Proteins." International Journal of Medicinal Chemistry 2017.1 (2017): 1529402. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
