Microscale Thermophoresis (MST)
Microscale thermophoresis (MST) is a powerful technique to quantify biomolecular interactions. It is based on thermophoresis, the directed movement of molecules in a temperature gradient, which strongly depends on a variety of molecular properties such as size, charge, hydration shell or conformation. Creative Biolabs is dedicated to establishing the most exquisite service platform for our clients and can provide comprehensive technical support for advancing our clients’ projects.
Description of the MST
MST technology is highly sensitive to virtually any change in molecular properties, allowing for precise quantification of molecular events independent of the size or nature of the investigated specimen. By combing the precision of fluorescence detection with the flexibility and sensitivity of thermophoresis, MST provides a flexible, robust and fast way to measure molecular interactions. During a MST experiment, a temperature gradient is induced by an infrared laser. The directed movement of molecules through the temperature gradient is detected and quantified using either covalently attached or intrinsic fluorophores. By combining the precision of fluorescence detection with the variability and sensitivity of thermophoresis, MST provides a flexible, robust and fast way to dissect molecular interactions.
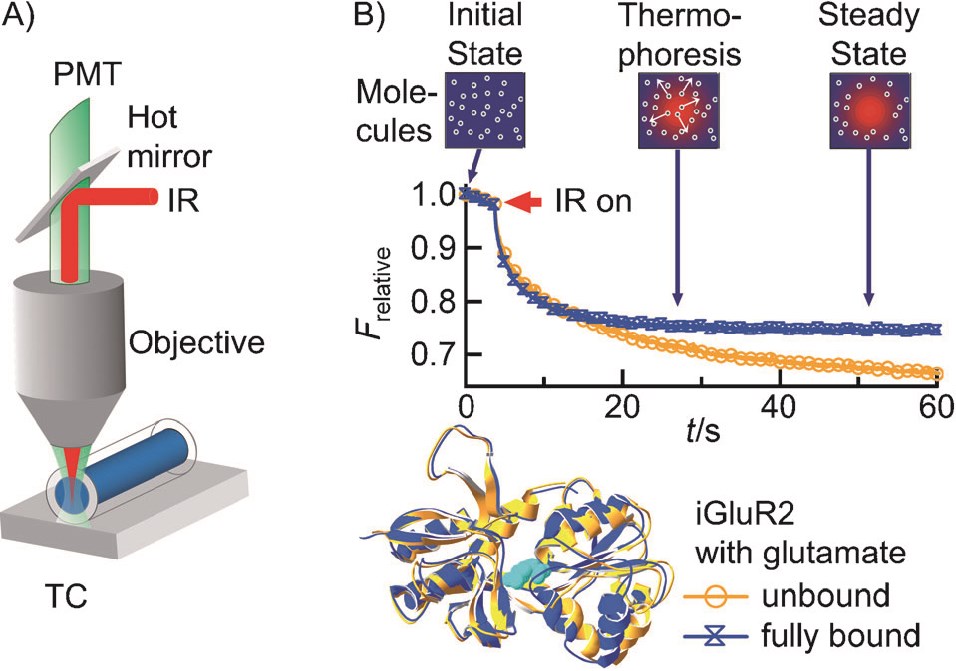 Fig.1 Label-free microscale thermophoresis.1
Fig.1 Label-free microscale thermophoresis.1
Services
MST can be used to investigate different aspects of biomolecular function. Creative Biolabs has established various protocols for the detailed characterization of biomolecules.
Detection of high-affinity interactions
The detection limit of MST can be pushed to low pM concentrations. MST has the unique benefit of detecting even minute changes in the hydration shell of a molecule, thereby making it suitable for analyzing a variety of interactions independent of the nature and size of the molecule.
Determination of binding states and stoichiometries
MST not only allows for a precise determination of binding constants, but can also be used to derive additional information about the molecular mechanism of the investigated interaction. For instance, MST can be used to discriminate between different binding modes, and can also be used to determine interaction stoichiometries.
Analysis of biomolecular interactions
MST is a powerful method to precisely determine Kds with low sample consumption and on a short time scale. It is well suited for thermodynamic analyses of molecular interactions. Binding between two interacting molecules has enthalpic and entropic components, corresponding to changes in both, structure and dynamics of each molecule. Both thermodynamic parameters are instrumental to elucidate the molecular mechanism of interaction. In addition, MST can also provide valuable information on the folding properties of proteins.
Advantages of MST
- Close to native conditions
- Affinity determination from 1 pM to mM
- No immobilization required
- Broad application range
- Measurement in complex bioliquids (serum, cell lysate)
- Purification free: fluorescent fusion proteins
Creative Biolabs is one of the world’s leading service providers for biophysics-based assays. Our state-of-the-art portfolio of biophysical technologies enables us to provide you with outstanding support. If you are interested in our services, please contact us for more details.
Reference
- Seidel, Susanne AI, et al. "Label-free microscale thermophoresis discriminates sites and affinity of protein–ligand binding." Angewandte Chemie (International ed. in English) 51.42 (2012): 10656. Distributed under Open Access license CC BY 2.5, without modification.
For Research Use Only.
