ALN-18328
Rare diseases that are observed to affect only a very small percentage of the population, have developed in significance and prominence in recent years. According to the record, there're approximately 7000 rare diseases, nearly 5% of which have approved treatment options. Hereditary transthyretin-mediated (hATTR) amyloidosis is a life-threatening disease resulted from the deposition of abnormal transthyretin protein. Remarkably, ALN-18328 (patisiran, ONPATTRO™) is a novel small interfering ribonucleic acid (siRNA) that has received marketing authorization and represents an excellent advance in the treatment of amyloidosis. This RNA interference (RNAi) therapeutic, is formulated in a lipid nanoparticle targeted to repress the production of hepatic transthyretin protein.
Introduction to hATTR Amyloidosis
Amyloid transthyretin amyloidosis (ATTR) is a progressive and normally fatal disease caused by the formation of mutated (hereditary ATTR [hATTR], also termed ATTR variant [ATTRv]) or normal transthyretin (wild-type ATTR) distributed throughout the body. The hATTR amyloidosis is known as an inherited, multisystem, and rapidly progressive disease induced by mutations in the transthyretin gene. The transthyretin protein is mainly produced in the liver and constitutes a tetramer to transport vitamin A (retinol) in correlation with retinol-binding proteins in the plasma and cerebrospinal fluid. So far, more than 120 transthyretin mutations have been identified and the most common is the valine (V)-to-methionine (M) mutation at the 30 position (V30M). The pathogenic transthyretin mutations allow misfolded transthyretin proteins that accumulate as amyloid deposits at lots of sites including heart, kidney, peripheral nerves, and gastrointestinal tract. This results in a heterogeneous clinical presentation such as sensory, motor, cardiomyopathy, autonomic polyneuropathy, and many other disease manifestations.
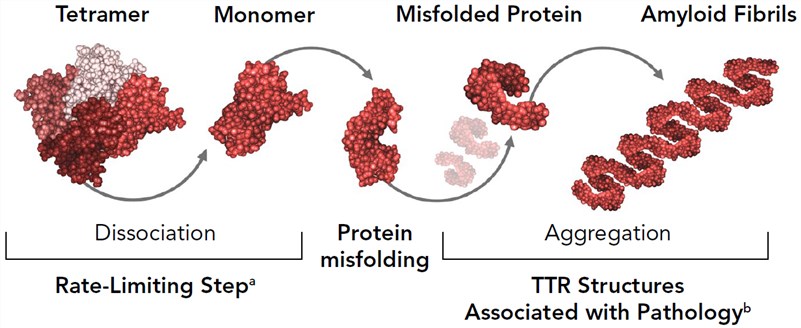 Figure 1. Mechanism of amyloid formation. (Gertz, 2019)
Figure 1. Mechanism of amyloid formation. (Gertz, 2019)
ALN-18328 in Clinical Trials
Available treatments for hATTR amyloidosis contain surgical intervention (liver transplant), transthyretin tetramer stabilizers (e.g. tafamidis, diflunisal), and transthyretin-reduction pharmacotherapies (e.g. inotersen, patisiran). Patisiran is a first-in-class RNAi therapeutics approved in the USA and EU to treat the polyneuropathy of hATTR amyloidosis in adults. The recommended concentration of the drug is 0.3 mg/kg administered through an intravenous infusion (over ~80 min) every three weeks, with a maximum limit dose of 30 mg for patients weighing over 100 kg.
ALN-18328 is a double-stranded siRNA encapsulated in a lipid nanoparticle. Lipid nanoparticle technology has been used as a delivery system for gene therapy and RNAi therapeutics to prevent oligonucleotides from the degradation by endogenous enzymes in the fluid and to facilitate targeted siRNA vehicles into the hepatocyte. In patisiran, the lipid nanoparticle consists of the siRNA (ALN-18328) and four lipid excipients, two of which have been applied in marketed drugs and two novel lipids (DLin-MC3-DMA and PEG2000-C-DMG), being employed as excipients for the first time. DLin-MC3-DMA is essential for particle formation, cellular uptake, fusogenicity, and endosomal release of siRNA into the cytoplasm of cells. PEG2000-C-DMG assists lipid nanoparticle stability in circulation after intravenous administration and offers optimum circulation time, hence enabling the uptake of ALN-18328 into the liver, the initial site of transthyretin production.
- Mechanism of action
siRNA molecule is the most attractive type of RNA-based therapeutic oligonucleotide drug, because their activities are catalytic and each siRNA is capable of inactivating certain target RNA molecules in a sequence-specific way. As a leading siRNA drug, ALN-18328 displays a unique mechanism of action that reduces the expression of the aberrant transthyretin protein that triggers hATTR amyloidosis, thereby solving the primary cause of the disease. By specifically binding to a genetically conserved sequence in the 3' untranslated region of mutant and wild-type transthyretin mRNA, ALN-18328 leads to its degradation via RNAi mechanism and then a decrease in serum transthyretin protein levels and tissue transthyretin protein deposits. The clinical development program for patisiran contains 6 stages of clinical studies. In Phase I and II studies, ALN-18328 plasma exposure increases in an about dose-proportional manner over the range of 0.01-0.5 mg/kg, resulting in a rapid, dose-dependent reduction of serum transthyretin synthesis.
Advancements in understanding and manipulating genes have laid the basis for researchers to alter the individual's genetic material to treat or prevent diseases. Over the past decades, siRNA-based therapeutics and drugs are some of the most important biopharmaceuticals that are in the commercial market as future medicines. At Creative Biolabs, we bring together top scientists, experts, and clinicians implicated in finding the latest drug targets, drug modalities, and innovative strategies for treating different rare diseases. If you want to know more about gene therapy or siRNA custom services, please directly contact us or send an e-mail with your specific request.
Reference
- Gertz, M.A.; et al. (2019). Advances in the treatment of hereditary transthyretin amyloidosis: A review. Brain Behav. 9(9): e01371. Distributed under Open Access license CC BY 4.0, without modification.
