CHD3 and Associated Diseases
The CHD3 gene, located at 17p13.1, consists of 7356 bp bases with 40 exons. The CHD3 protein has an ATP-binding helicase region, 2 finger-plant homology domains, and 2 chromatin domains.
Function and Structure of CHD3
CHD3 encodes chromatin domain-helicase-DNA-binding protein 3, which is a member of the mammalian CHDs family. All proteins in this family contain two helicase domains and two chromatin domains, which together play key roles in transcriptional regulation and chromatin remodeling during mammalian development. Based on functional domain differences, CHD3 is classified as a class II CHD protein, which contains two additional plant homology domains responsible for physically binding CHD to nucleosome remodeling deacetylases and forming the chromatin remodeling complex, a process that plays a key role in embryonic development. In addition, CHD3 also retains a domain with unknown significance.
The CHD3 protein moves nucleosomes to restrict DNA into tight packages to help remodel chromatin, which in turn regulates gene activity and expression. In addition, CHD3 exerts its function by forming the core ATP subunit of the nucleosome remodeling and deacetylase (NuRD) complex, and then participates in chromatin remodeling through the histone deacetylation mechanism to regulate gene expression at the Spatio-temporal level. During this process, ATP is broken down to provide energy for DNA remodeling. Through its ability to regulate gene activity and maintain DNA structure/integrity, CHD3 is involved in numerous processes during embryonic development, determining cell fate and controlling the cell cycle. Some studies have also proven the correlation between CHD3 and the development and migration of neurons.
CHD3 and Snijders Blok-Campeau Syndrome
Compared with other members of the CHD family, the specific syndromes associated with CHD3 mutations have not been well studied and characterized. However, in recent years, a Snijders Blok-Campeau syndrome characterized by language impairment, macrocephaly, and intellectual disability has been reported, and CHD3 mutation has been identified as the main causative factor for this symptom. Research has demonstrated a direct relationship between more than 25 CHD3 mutations and this disease. These mutations alter individual protein building blocks in CHD3, affecting domains involved in the breakdown of ATP, and thereby interfering with the energy supply during chromatin remodeling. Mutations in CHD3 increase or decrease chromatin remodeling activity, affecting the activity of many genes that direct organ and tissue development. Its specific molecular mechanism is still unknown, but it is presumed to be related to the formation of NuRD complexes.
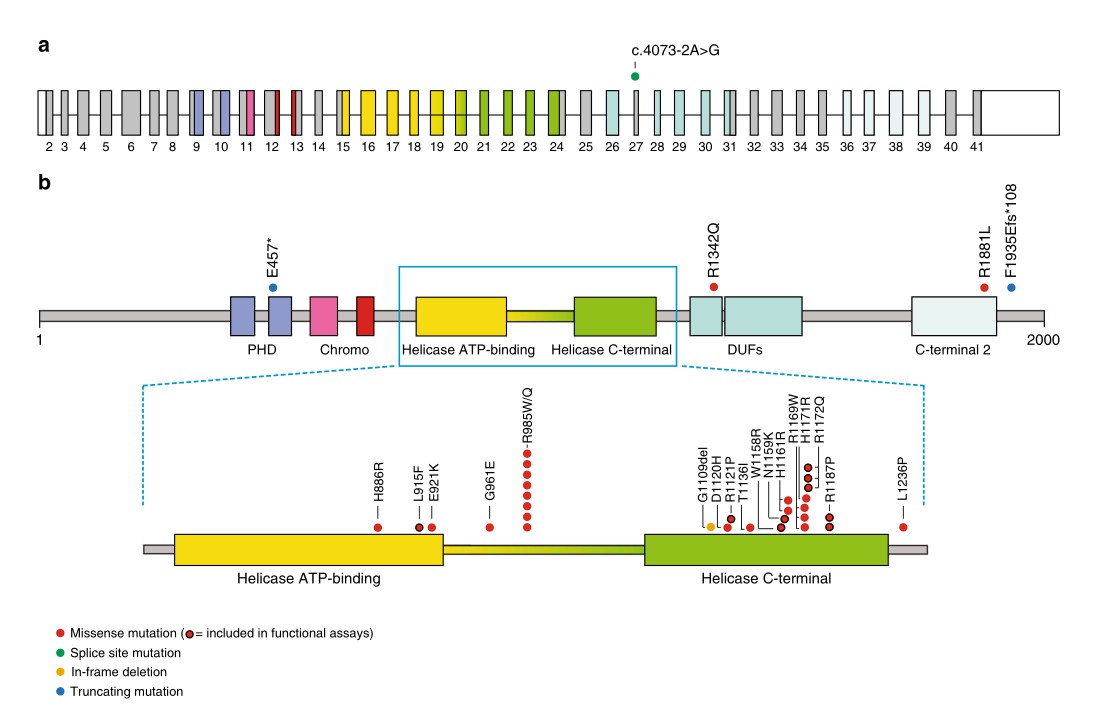 Fig 2. Schematic view of CHD3 transcript and protein with de novo mutations. (Blok, 2018)
Fig 2. Schematic view of CHD3 transcript and protein with de novo mutations. (Blok, 2018)
CHD3 and Other Diseases
Chromatin remodeling is critical during brain development, so mutations in CHD3 are also being genetically analyzed as possible neurological/encephalopathy causative factors. CHD3 mutants may result in markedly reduced or increased ATPase activity and a severe reduction in chromosomal remodeling capacity, but it is unclear how enzyme inactivation and activating mutations have devastating effects on the neurodevelopmental system. In addition to Snijders Blok-Campeau syndrome, mutations in CHD3 are also potential pathological factors for symptoms such as dermatomyositis, stuttering, and apraxia. The role of CHD3 mutation in various diseases still needs to be further explored.
Thanks to our strong molecular biology technology platform, Creative Biolabs provides one-stop solution services for the CHD3 gene and related proteins. Whether you are willing to elucidate the structure and function of CHD3 or further explore its role in various disease processes, we will be your best choice.
Reference
- Blok, L.S.; et al. CHD3 helicase domain mutations cause a neurodevelopmental syndrome with macrocephaly and impaired speech and language. Nature Communications. 2018, 9: 4619. Distributed under Open Access license CC BY 4.0, without modification.
