RB1 and Associated Diseases
The retinoblastoma susceptibility gene (RB1) is the first human gene molecularly defined as a tumor suppressor gene. Its discovery and characterization represent that cancer research has entered the field of genetics, and it has opened up a new path for understanding human genes and preventing cancer.
RB1 in Tumor
Mutations in RB1 are found in nearly all familial/sporadic retinoblastomas, and have also been observed with varying frequencies in other human cancers. RB1 deficiency during malignant development has been demonstrated by genome sequencing in breast cancer, non-small cell lung cancer, chronic myelogenous leukemia, bladder cancer, glioma, osteosarcoma, and esophageal cancer. Transcriptional signatures associated with RB1 mutations include upregulation of many genes required for cell proliferation, whose loss results in the inability of cells to exit the native cycle, impairs G1/S arrest, slows cellular senescence and forces entry into the cell division cycle.
RB1, pRB and E2F
pRB, the gene product of RB1, acts as a negative regulator of cell proliferation in cells, and its main function is to limit the transcription of cell cycle-related genes through direct regulation of E2F transcription factors. The activity of pRB is controlled by cyclin-dependent kinases (CDKs), and activated pRB inhibits the transcription of E2F, thereby limiting the expression of cell proliferation-related genes. The function of pRB is damaged by RB1 mutation or hyperphosphorylation during the pathogenesis of many tumors. Inactivation of pRB will cause the cells to fail to exit the cell cycle smoothly and stay in a state that is extremely prone to oncogenic proliferation for a long time. pRB hyperphosphorylation experiments also confirmed this theory.
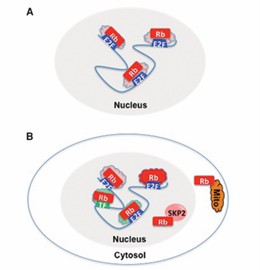 Fig 1. Multiple mechanisms of action of RB1. (Dyson, 2016)
Fig 1. Multiple mechanisms of action of RB1. (Dyson, 2016)
RB1 mainly affects the surrounding environment of tumor cells in an E2F-dependent manner, including the secretion of cytokines, immune cell responses and even the behavior of distant cells. pRB recruits and balance the transcription-repressing RB1/E2Fs/DPs complex, which represses the transcription of target genes by activating histone deacetylases. E2F liberated by RB1 inactivation recruits histone acetylases to transactivate genes responsible for cell cycle progression, DNA damage repair, DNA synthesis, chromatin structure homeostasis, and apoptosis, particularly cyclin E and CDK2. These activated genes and the upregulation of their expression severely disrupt the cell's ability to exit the cell cycle, leaving the cell in an undifferentiated, oncogenic state.
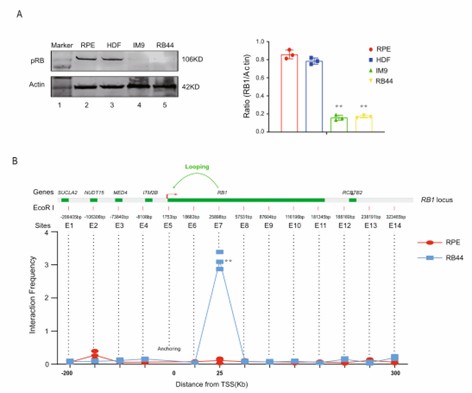 Fig 2. pRB abundance and effect on chromosome conformation. (Wen, 2022)
Fig 2. pRB abundance and effect on chromosome conformation. (Wen, 2022)
RB1 and Other Factors
In addition to E2F, pRB also has a sub-exponential relationship with a large number of proteins, and more than 300 proteins are directly affected by RB1 or pRB, including cell cycle proteins, signaling molecules, chromatin modifiers and tissue-specific transcription factors. For example, studies have shown that after RB1 loss, Aurora A and polo-like kinase 1 (PLK1) are upregulated, which may lead to aneuploidy or other mitotic abnormalities, which further lead to malignant progression. More than 14 RB1s with different mono-acidification states have been verified, and this complex state greatly increases the complexity of the interaction relationship with specific binding partners.
In fact, with the in-depth development of biological tools and sequencing experiments, researchers found that the role of RB1 in inhibiting the malignant progression of cancer cannot be explained only by its function of limiting cell cycle progression. More in-depth investigations revealed more roles of RB1 in cancer pathogenesis, for example, RB1 controls the transcription of androgen receptor (AR) through the E2F family of transcription factors and enhances cancer resistance to endocrine therapy. The inactivation of RB1 affects IL-6 and transcriptional activator 3, thereby enhancing cellular malignant progression and stem cell-like behavior. Loss of RB1 in breast cancer cells activates AMPK, phosphorylates acetyl-CoA carboxylase, which in turn activates fatty acid oxidation, resulting in cells acquiring undifferentiated features.
Creative Biolabs provides customers with standardized biological or molecular biology experiments including gene editing, gene sequencing, protein detection, etc. With our help, you may explore the molecular relationship between RB1 and associated diseases on a deeper and broader level. Please feel free to contact us.
References
- Dyson, N.J. RB1: a prototype tumor suppressor and an enigma. Genes and Development. 2016, 30: 1459-1502. Distributed under Open Access license CC BY 4.0, without modification.
- Wen, X.; et al. Interruption of aberrant chromatin looping is required for regenerating RB1 function and suppressing tumorigenesis. Communications Biology. 2022, 5: 1036. Distributed under Open Access license CC BY 4.0, without modification.
