KDM5A and Associated Diseases
Lysine-specific demethylase 5A (KDM5A) is responsible for the removal of the second and third methyl groups on lysine 4 of histone H3. This modification regulates epigenetics, affects post-translational chromatin modifications, maintains genome stability, and affects a wide range of nuclear events. KDM5A regulates transcription and intervenes in many pathological/physiological cellular events through demethylase-dependent/independent pathways in disease or homeostasis circumstances.
Function of KDM5A
The main and most widely studied function of KDM5A is that it rearranges with NUP98 to generate the fusion gene NUP98KDM5A and demethylates H3K4me2/3, thereby participating in DNA damage response, mediating blood cell proliferation and erythroid differentiation. Furthermore, as a general transcriptional co-repressor, KDM5A represses Wnt/β-catenin activity and blocks the WNT6 gene promoter demethylase-dependently, which leads to enhanced differentiation of preadipocyte. KDM5A is also observed in many critical cellular events, including stem cell differentiation, reprogramming, cell cycle, circadian rhythm, NK cell activation, etc. Some examples of the proven role of KDM5A in vivo are listed in the table below.
| Tissues/Cells | Functions | Mechanism |
| IMR-90 cells | Regulate cellular senescence | Involved in retinoblastoma-mediated gene silencing |
| NK cells | Activating NK cells | Interacts with p50 and inhibits SOCS1 |
| Preadipocytes | Promote differentiation |
Blocks WNT/β-catenin activity Inhibits WNT6 transcription demethylase-dependently |
| Reprogramming-resistant fibroblasts | Inhibits the reprogramming process | Repression of OCT4 expression by KDM5A transcription |
| Mice embryonic stem cells | Affect cell differentiation | Repression of cell cycle-related gene expression by KDM5A transcription |
| Heart |
Affects the circadian clock Activates CLOCK-BMAL1 |
Interacts with CLOCK-BMAL1 Increases histone acetylation and enhances transcription of related genes |
KDM5A and AML
AML is a highly fatal cancer that is common in the elderly. The certain domain of KDM5A interacts with NUP98. The fused gene blocks myeloblast differentiation and ultimately leads to leukemia. There is evidence that this domain may be located at the C-terminal of KDM5A. KDM5ANUP98 promotes the over-expression of Hoxa gene clusters by binding to Hoxa gene promoter specifically, leading to the repression of epigenetic systems required for normal cell differentiation.
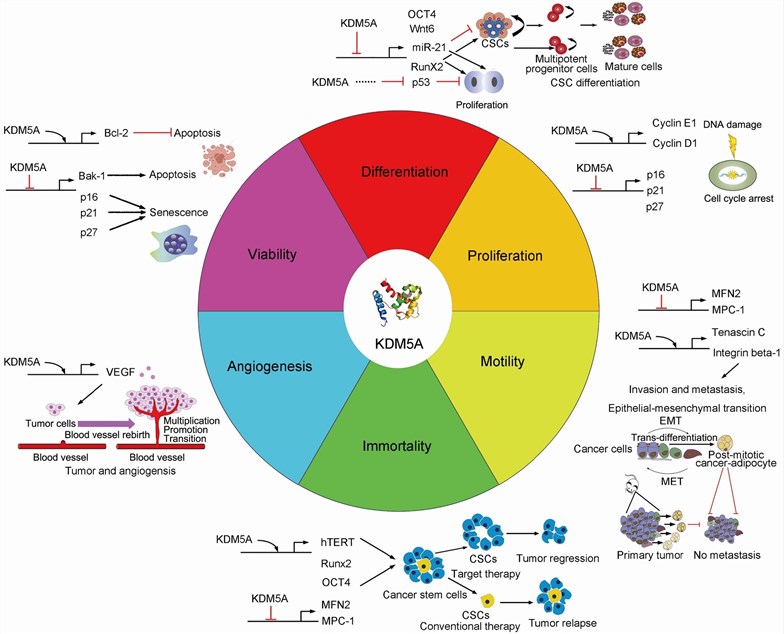 Fig 1. Biological function and mechanism of KDM5A. (Yang, 2021)
Fig 1. Biological function and mechanism of KDM5A. (Yang, 2021)
KDM5A and Lung Cancer
Clinical statistics show that KDM5A is highly expressed in lung cancer patients and promotes the pathogenesis and drug resistance of lung cancer cells. Akt can interact with KDM5A, a process that affects a series of downstream pathways including HIF-1α-VEGF and ultimately leads to vascular tissue formation. The interaction of Akt and KDM5A also leads to changes in the levels of E-cadherin and N-cadherin (the former inhibits and the latter up-regulates). Altered levels of cadherin induce epithelial to transform into mesenchymal in lung cancer tissues. Among other things, KDM5A also interacts p27, ITGB1 and Cyclin D1 with their promoters to manipulate the transcription process. Downregulation of p27 and upregulation of others lead to the pathogenesis of lung cancer.
KDM5A and Osteosarcoma
KDM5A is highly expressed in osteosarcoma and its surrounding tissues, and it participates in osteosarcoma's invasion and metastasis through IL6/JAK/STAT3 and TNF-α/NF-κB pathways. Knocking out or interfering with KDM5A expression increases the level of p27 and down-regulates Cyclin D1, thereby inhibiting osteosarcoma cells’ proliferation and controlling the growth of tumors.
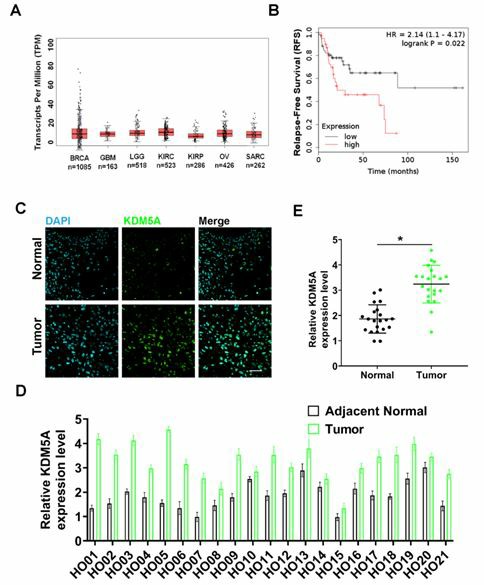 Fig 2. KDM5A in human osteosarcoma cancer tissues. (Peng, 2021)
Fig 2. KDM5A in human osteosarcoma cancer tissues. (Peng, 2021)
KDM5A in Other Diseases
Clinical statistics prove the correlation of KDM5A with a series of other diseases, such as autism, renal failure, various infections or congenital heart disease. In addition, KDM5A also has an influence on many types of cancer, including glioblastoma, pancreatic cancer, renal cell carcinoma, breast cancer, etc.
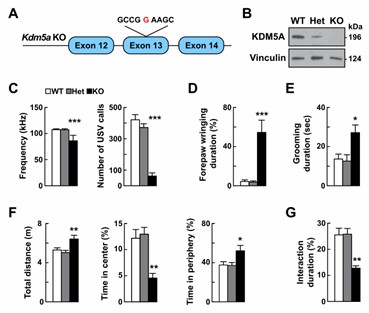 Fig 3. KDM5A dysfunction leads to learning and memory deficits. (Hayek, 2020)
Fig 3. KDM5A dysfunction leads to learning and memory deficits. (Hayek, 2020)
Creative Biolabs is committed to providing the most robust and comprehensive KDM5A solutions for clients all over the world. Our high-quality services including gene sequencing, protein analysis, and signaling pathway research will help you better explore the role of KDM5A physiology and pathology. Please feel free to contact us.
References
- Yang, G.J.; et al. The emerging role of KDM5A in human cancer. Journal of Hematology and Oncology. 2021, 14: 30. Distributed under Open Access license CC BY 4.0, without modification.
- Peng, D.; et al. Histone demethylase KDM5A promotes tumorigenesis of osteosarcoma tumor. Cell Death Discovery. 2021, 7: 9. Distributed under Open Access license CC BY 4.0, without modification.
- Hayek, L.E.; et al. KDM5A mutations identified in autism spectrum disorder using forward genetics. Elife. 2020, 9: e56883. Distributed under Open Access license CC BY 4.0, without modification.
