TNPO1 and Associated Diseases
Transportin-1 (TRN-1), encoded by the TNPO1 gene, is a nuclear import receptor. TRN-1 plays an integral role in regulating the nuclear import cycle of cytoplasmic proteins. At present, studies have found that TRN-1 is closely related to the pathogenesis of Hutchinson-Gilford progeria syndrome (HGPS) and amyotrophic lateral sclerosis (ALS). Creative Biolabs has established various leading biotechnology platforms to make it easier to develop drugs. We can provide one-stop services from design ideas to result evaluation, and look forward to enabling you to discover drugs that precisely target genes.
Important Function of TNPO1
The TNPO1 gene is located on human chromosome 5 (5q13) and contains 26 exons. TRN-1, encoded by the TNPO1 gene, is an important nuclear import receptor β (karyopherin-β1, Kapβ) family member. Therefore, TRN-1 is also known as Kapβ2. The protein structure of TRN-1 consists of 20 HEAT repeats in a supercoiled form. The two arches each consist of 13 HEAT repeats, while the overlapping portion of the two arches consists of 5 HEAT repeats. These two arches are recognized by nuclear localization sequence (NLS) and RanGTP, respectively. The binding of TRN-1 and the cytoplasmic protein-NLS can guide the cytoplasmic protein into the nuclear pore complex (NPC) to promote the nuclear entry of the protein. Subsequently, Ran-GTP can bind to TRN-1 to further promote the release of cytoplasmic proteins into the nucleus. TRN-1-RanGTP returned to the cytoplasm can bind to RanGAP and RanBPs, which leads to the dissociation of RanGDP. After the nuclear import of cytoplasmic proteins is complete, TRN-1 waits for the next task. In eukaryotic cells, TRN-1 is mainly responsible for transporting a large number of RNA-binding proteins (RBPs) involved in gene transcription, such as hnRNP family proteins, to the nucleus. In addition, studies have found that TRN-1 is involved in the regulation of nuclear import of viral genome and capsid proteins, a combination of RNA and RBPs, and cargo transport within immobile cilia.
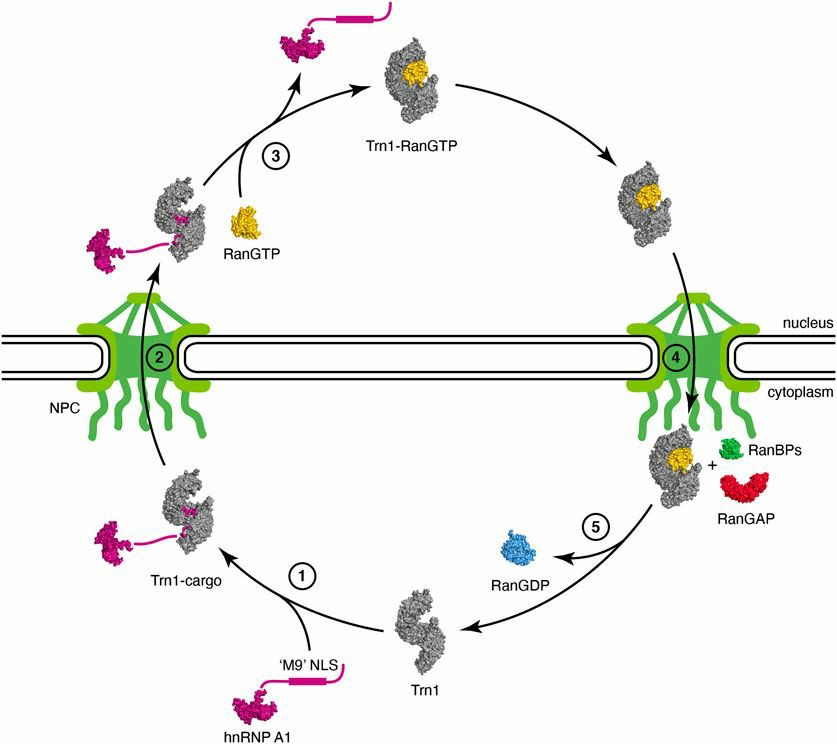 Fig.1 Nuclear import cycle of Transportin-1. (Mboukou, 2021)
Fig.1 Nuclear import cycle of Transportin-1. (Mboukou, 2021)
TNPO1-related Diseases
HGPS is a rare genetic disorder. Compared with healthy people, the body of HGPS patients ages very quickly, showing abnormal development of multiple organs, general weight loss, and baldness. In fibroblasts from patients with HGPS, microtubule immobilization can inhibit the conversion of TRN-1-RanGTP to TRN-1, which leads to the failure of TRN-1 to participate in the nuclear import cycle of cytoplasmic proteins. Difficulty in the nuclear import of cytoplasmic proteins hinders the expression of a large number of genes and drives cells into a senescent state. Therefore, the repositioning of TRN-1 is a promising therapeutic strategy for HGPS.
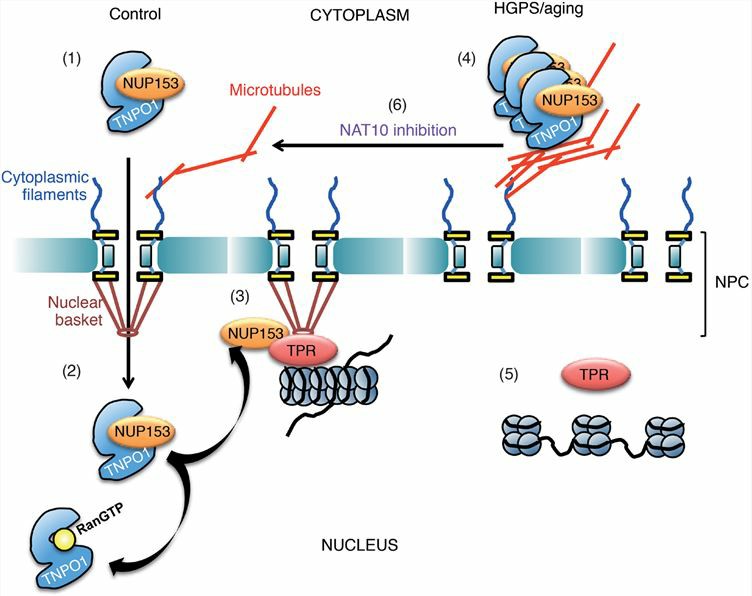 Fig.2 Model for how NAT10 inhibition rescues HGPS phenotypes. (Larrieu, 2018)
Fig.2 Model for how NAT10 inhibition rescues HGPS phenotypes. (Larrieu, 2018)
The mutation of FUsed in Sarcoma (FUS) is one of the pathogenic factors of ALS. Under normal physiological conditions, FUS is mainly localized in the nucleus. But mutated FUS stays in the cytoplasm and forms inclusion bodies. Therefore, the mislocalization of FUS in cells may be an important factor associated with ALS disease. Studies have found that TRN-1 plays a key role in regulating the nuclear import of the FUS C-terminal nuclear localization sequence (FUS-NLS). Mutant FUS can prevent TRN-1 from binding to FUS-NLS, and FUS accumulates in the cytoplasm. Therefore, TRN-1 may be one of the key targets for the treatment of ALS.
Creative Biolabs continues to provide customers with high-quality products and services related to viral vectors and nucleic acid interference. If you have research ideas about the TNPO1 gene, please contact us without hesitation. Our professional talents will provide you with high-quality solutions and services.
References
- Mboukou, A.; et al. Transportin-1: a nuclear import receptor with moonlighting functions. Frontiers in Molecular Biosciences. 2021, 8:638149. Distributed under Open Access license CC BY 4.0, without modification.
- Larrieu, D.; et al. Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway. Science Signaling. 2018, 11(537). Distributed under Open Access license CC BY 4.0, without modification.
