PTPRF and Associated Diseases
Protein tyrosine phosphatase, receptor type F (PTPRF) is a phosphatase that participates in many cellular physiological behaviors and plays a role in tumor genesis.
Structure of PTPRF
PTPRF is collectively classified into the LAR-RPTP subfamily due to its similar signaling mechanisms and structures with PTPRD and PTPRS. The extramembrane domain of PTPRF realizes extracellular molecular recognition and specific interaction by alternative splicing or DNA glycosylation process to produce specific splicing isoforms. The proximal PTP domain of the inner membrane, D1, is responsible for catalyzing the phosphorylation of tyrosine. The PTP domain (D2) can dock with certain molecules, but D2 does not have phosphatase activity without the substitution of specific residues.
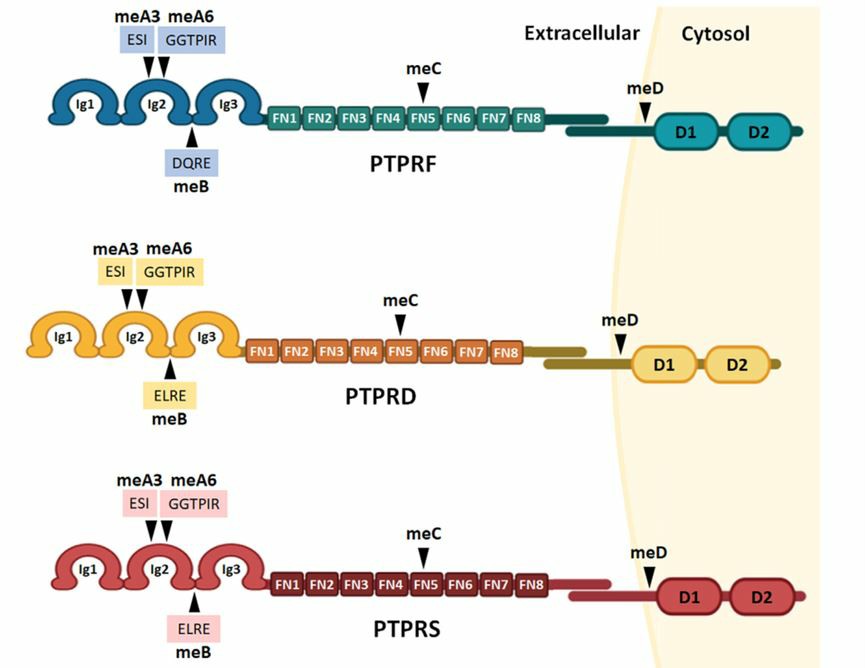 Fig 1. PTPRF, PTPRS, and PTPRD with similar protein structures. (Cornejo, 2021)
Fig 1. PTPRF, PTPRS, and PTPRD with similar protein structures. (Cornejo, 2021)
Function of PTPRF
PTPRF has a dual function. It can participate in cell adhesion and other cellular functions through the extramembrane structure, while its influence on cell signaling processes is achieved through the catalytic activity of its intramembrane domain. Reversible phosphorylation and dephosphorylation processes are essential for the control of cellular processes. PTPRF has been shown to regulate the phosphorylation status of at least 205 different proteins involved in cellular processes including cytoskeleton formation, cell adhesion, etc.
Another important function of PTPRF is to affect synaptic differentiation and excitatory signal transmission in various ways. Exons of PTPRF can act as ligands to directly induce PTPRF phosphatase activity and activate downstream pathways. Presynaptic PTPRF can indirectly participate in LTD through binding NGL-3 or regulating AMPAR synaptic transmission through the interaction with SALM5, and these molecules will promote and induce synaptic differentiation. In addition to NGL-3 and SALM3/5, a cis-interaction of PTPRF with netrin-G1 was observed, a process that also induces excitatory differentiation in a unidirectional fashion.
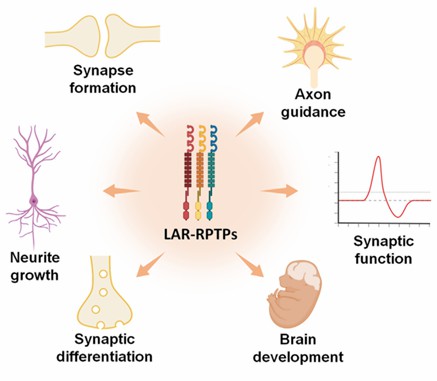 Fig 2. PTPRF and LAR-RPTPs participate in several neural functions. (Cornejo, 2021)
Fig 2. PTPRF and LAR-RPTPs participate in several neural functions. (Cornejo, 2021)
PTPRF in Cancer
Gene sequencing has confirmed the correlation between PTPRF and various tumor diseases. Studies on gastric adenocarcinoma have shown that PTPRD mediates the phosphorylation of signal-regulated kinase 1/2, which significantly inhibits its downstream signal intensity, which ultimately leads to the blockage of tumor invasion.
Gene knockout experiments in colorectal cancer tissue proved that PTPRF is an upstream signaling molecule of the β-catenin complex and directly affects the activation of Wnt signaling pathway. Restricted PTPRF level will lead to a decrease in the expression of cancer stem cell-related genes, and a significant increase in the number of differentiated tumor cells.
PTPRF in Non-Cancer Diseases
Many of the conditions directly associated with PTPRF are systemic dysfunctions such as metabolic diseases, colitis or cancer, however, there is also evidence that PTPRF regulates the pathogenesis of neurological diseases by affecting synaptic interacting proteins. The lower level of PTPRF and reduced enzyme activity observed in Huntington's disease patients lead to reduced focal adhesion of cells and defects in cell-cell adhesion, which is often accompanied by neurological dysfunction and cell death. In patients with neuronal lipofuscinopathy, loss of soluble lysosomal palmitoyl thioesterase causes repression of PTPRF expression and cytoskeletal damage, which constitutes an important part of the pathogenesis of neurodegeneration. In immune-mediated demyelinating diseases, the expression of PTPRF was upregulated in exosomes obtained from the cerebrospinal fluid of patients, which was directly associated with the development of demyelinating diseases and proposed as a biomarker for its early diagnosis. Furthermore, studies in animal models have shown that PTPRF plays an important role in axon regeneration, hippocampal neurogenesis, and basal forebrain cholinergic signaling.
Years of experience in biological research make Creative Biolabs confident to be your best partner in exploring the molecular mechanism of PTPRF. Our excellent experimental platform and R&D team will fulfill all your needs whether you want to explore the pathological mechanism of PTPRF through standardized general molecular biology assays, or optimize and improve existing experimental procedures to achieve further goals. Please feel free to contact us.
Reference
- Cornejo, F.; et al. LAR receptor tyrosine phosphatase family in healthy and diseased brain. Frontiers in Cell and Developmental Biology. 2021, 9: 659951. Distributed under Open Access license CC BY 4.0, without modification.
