RANBP2 and Associated Diseases
Ran-binding protein 2 (RANBP2) is one of the main component proteins of cytoplasmic filaments in the nuclear pore complex, which is widely present on the cell membrane and is responsible for many key cellular activities.
Structure and Function of RANBP2
RANBP2 is a RanGTP-binding protein located on the nuclear membrane, which has multiple domains, including a leucine-rich domain at the N-terminus, a short cyclophilin homology domain at the C-terminus, and a small ubiquitin-like modifier E3 ligase domain. The E3 domain interacts with ubiquitin-conjugating enzyme 9 and SUMO-modified Ran GTPase activating protein (SUMO-RanGAP1), a process that is critical for the formation of cytoplasmic filaments. The most deeply studied function of RANBP2 is to mediate sumoylation. In addition, as the main protein on the nuclear membrane, RANBP2 is also involved in many important cellular processes, including photoreceptor transport, glucose metabolism, mitosis, mRNA metabolism, nucleoplasm transport and myogenesis. It has also been shown that RANBP2 can form cytosolic aggregates outside the nuclear pores.
RANBP2 in Acute Necrotic Encephalopathy (ANE)
ANE1 is a rare pediatric neurological disorder that is usually triggered by viral infections such as influenza and causes loss of consciousness. Clinical information on ANE1 confirmed a direct relationship between missense mutations in RANBP2 and the pathogenesis of ANE. Genetic testing reports show that about 75% of familial/recurrent ANE cases carry missense RANBP2 genes.
There is no conclusion about the specific mechanism of RANBP2 mutation in the pathogenesis of ANE1, but there are several feasible hypotheses. A speculative explanation is that the mutation of RANBP2 will lead to mitochondrial metabolic disorders, especially the association with mitochondrial cytochrome c oxidase assembly protein COX11 will be disturbed, thereby affecting the cellular metabolism of glucose. Another widely accepted hypothesis is that mutations in RANBP2 directly lead to cytokine storms that induce glial and neuronal apoptosis and lead to neurological disorders.
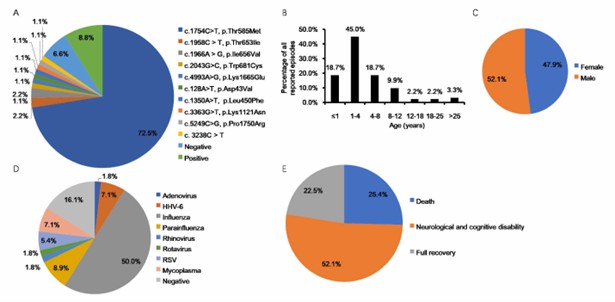 Fig 1. Statistical analysis of correlation between clinical information and RANBP2 mutation in acute necrotizing encephalopathy. (Jiang, 2022)
Fig 1. Statistical analysis of correlation between clinical information and RANBP2 mutation in acute necrotizing encephalopathy. (Jiang, 2022)
RANBP2 in Virus Infection
The interaction between RANBP2 and viral proteins and antiviral host factors can change or directly manipulate the infection process of viruses. RANBP2 is considered to be necessary for many viruses to enter the nucleus to start the process of replication and assembly. Inhibition of RANBP2 can reduce the number of viruses entering the nucleus, thereby inhibiting virus replication and expression of viral proteins. Some widely studied viruses and their relationship with RANBP2 are listed below.
| Virus | Family | Consequences |
| Adenoviruses | Adenoviridae | Interfering with the nuclear envelope, facilitate entry of viral DNA into the nucleus |
| V ACV | Poxviridae |
Maintain the size and number of virus factories Increase virus production |
| HSV-1 | Herpesviridae |
Facilitates attachment of HSV-1 capsid to nuclear surfaces Reduced levels of O-glycosylated RanBP2 |
| BPV | Papillomaviridae | Facilitates the import of viral protein E1 into the nucleus |
| SARS-CoV-2 | Coronaviridae | Downregulates the expression level of RanBP2 and possibly promotes the development of a "cytokine storm" in the most severe patients with COVID-19 |
| Flaviviridae | HCV |
Increased mRNA and protein levels of RanBP2 May contribute to HCV viral immune evasion, assembly, and replication |
| HRV | Picornaviridae |
Degrades RanBP2 Disruption of nuclear envelope permeability Interfering with nuclear-cytoplasmic transport |
| Retroviridae | HIV-1 |
Facilitates rapid nuclear import of HIV-1 pre-integration complexes Facilitates viral infection and evades innate immune sensors |
Here at Creative Biolabs, we help our clients better and more deeply explore the intrinsic molecular link between RANBP2 and associated diseases. Our comprehensive, flexible and stable service will be your best choice to explore the function of the RANBP2 molecule under physiological and pathological conditions. Please feel free to contact us.
Reference
- Ogawa, Y.; et al. Endogenously expressed RANBP2 is not at the axon initial segment. Journal of Cell Science. 2021, 134: 256180. Distributed under Open Access license CC BY 4.0, without modification.
