Lentiviral Vector Design Services for Gene Silencing
Gene silencing by RNA interference (RNAi) has become a powerful and rapidly evolving method for investigating gene function in mammalian cells, especially small interfering RNA (siRNA) and short-hairpin RNA (shRNA). Lentiviral vectors (LVs) provide a unique tool to integrate these RNA expression cassettes with the goal of locally knocking down the expression of specific genes to assess the function of a gene. Creative Biolabs has proprietary technology and versatile platform in viral vector design, which enables us to provide excellent gene silencing LVs design services for our customers.
Gene Silencing Introduction
RNAi, an RNA-mediated gene silencing mechanism, has recently emerged as a novel pathway that allows modulation of gene expression. As a tool in mammalian cell systems, gene silencing is induced by the delivery of a double-stranded RNA (dsRNA) that matches the mRNA target sequence. The dsRNA can be delivered as a siRNA via transfection, or shRNA via transfection or viral delivery of a plasmid. Because siRNA or shRNA can suppress the expression of genes of interest, RNAi-mediated gene silencing has become an essential technology to study gene functions in mammalian cells. To exploit this technique, efficient siRNA/shRNA delivery methods must be developed. Studies have found that LVs can be engineered to achieve stable and efficient gene silencing in a variety of cells. In gene-silencing LVs, siRNA can be delivered as a form of shRNA driven by RNA polymerase III promoters, or as a part of a miRNA-like structure expressed from RNA polymerase II promoter. Nowadays, gene-silencing LVs have been successfully used to inhibit HIV-1 infection, delay the occurrence and progression of amyotrophic lateral sclerosis in mice, prolong the survival rate of mice with scrapie development and increase the expression of fetal hemoglobin.
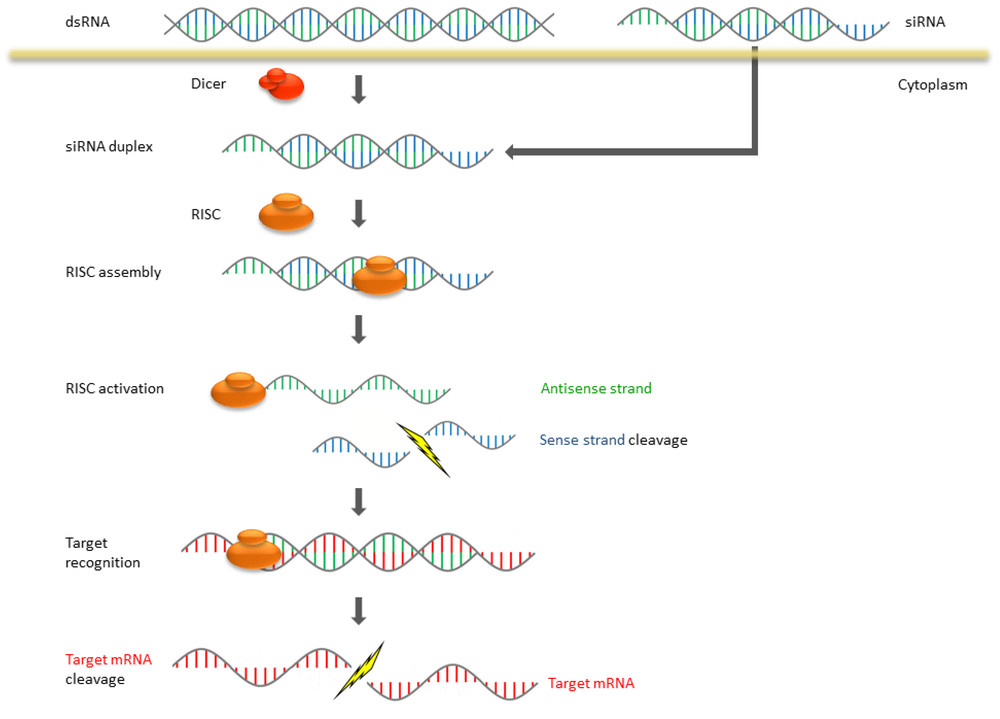 Figure 1. Mechanism of RNA interference (RNAi) in mammalian systems.1
Figure 1. Mechanism of RNA interference (RNAi) in mammalian systems.1
Lentiviral Vector Overview
Lentiviral vectors are multi-component gene delivery vehicles that are based on the lentivirus family of viruses, most commonly the human immunodeficiency virus type 1 (HIV-1). In contrast to simple retroviruses, lentiviruses can transduce non-dividing cells. This is due to the ability of the virus to actively carry the pre-integration complex across the nuclear envelope. This feature is critical for in vitro and in vivo targeting of terminally differentiated non-dividing cells such as neurons or hematopoietic stem cells.
Lentiviral Vector Design Step for Gene Silencing
-
Step 1
Target Validation and Silencer Design
In silico analysis: Use proprietary algorithms (e.g., CRISPRme for off-target prediction) to identify optimal shRNA/amiRNA sequences, avoiding regions with single nucleotide polymorphisms (SNPs) or homology to essential genes.
Minimum free energy (MFE) calculation: Ensure the silencer hairpin has an MFE of -20 to -30 kcal/mol, which is critical for stable folding and processing.
-
Step 2
Backbone Customization
Promoter Selection: Choose between constitutive (e.g., EF1α, PGK) or inducible (e.g., Tet-On/Tet-Off) promoters based on customer needs. For tissue-specific silencing, we offer promoters for albumin (hepatocytes), synapsin I (neurons), or CD4 (T cells).
Safety Modifications:
Insulators (e.g., cHS4): Reduce positional effects (variable behavior due to integration location).
Suicide genes (e.g., herpes simplex virus thymidine kinase, HSV-TK): Enable elimination of transduced cells in the event of adverse reactions.
-
Step 3
Pseudotyping and Production Optimization
- Envelope selection: Match the pseudotype to the target cell (e.g., VSV-g for broad-spectrum, RD114 for hematopoietic cells).
- Production: Use a serum-free suspension cell line (e.g., HEK293T) to produce vector at titers >10⁸ TU/mL, validated by qPCR and flow cytometry.
What Is a Technology That Silences Genes?
Integrating lentiviral vectors expressing shRNAs represent one of the most powerful and widely adopted technologies for silencing genes for stable knockdown studies.
Functional Genomics Screening
High-throughput shRNA libraries packaged in lentiviral vectors are indispensable for genome-wide functional screens. For example, a pooled lentiviral shRNA library can be used to infect cells, followed by a selection process (e.g., drug resistance, survival in a specific culture medium). Sequencing the integrated shRNAs in surviving cell populations (termed shRNA-Seq) can identify genes whose knockdown confers a selective advantage, thereby identifying novel drug targets or essential survival pathways (e.g., identifying genes involved in chemotherapy resistance in cancer models).
In Vivo Target Validation
The stability and high efficiency of lentiviral vectors make them ideal for creating stable knockout cell lines or for direct in vivo delivery (e.g., stereotactic injection into the brain). A landmark example involved silencing the huntingtin (HTT) gene in a rodent model of Huntington's disease, demonstrating the therapeutic potential of mRNA knockdown in neurodegenerative diseases.
Core Services at Creative Biolabs
Using in-house developed protocols, Creative Biolabs provides unique gene silencing LVs design which allows efficient expression of any siRNA by transfection or lentiviral infection of target cells. The scheme is based on a unique convergent promoter design to increase the efficiency of target gene knockdown without the need for a hairpin loop structure commonly used for single promoter vectors. Our protocol combines the specificity of siRNA-mediated silencing cassettes with the versatility of LVs to stably transduce a wide range of cell types. The combination of the lentiviral and siRNA technologies provides a powerful tool to achieve long-term down-regulation of specific target genes both in vitro and in vivo.
- Custom Vector Design
- Preclinical Production
- Custom shRNA Lentivirus Library
- In Vitro Validation
- In Vivo Validation
- Custom shRNA Lentivirus Service
Technical Performance Metrics of Creative Biolabs' Lentiviral Vectors
| Performance Parameter | Validation Method | Documented Results |
|---|---|---|
| Vector Construction Accuracy | Restriction digestion, colony PCR, sequencing | Correct insert orientation and sequence confirmed |
| Gene Silencing Efficiency | qRT-PCR, Western blot | mRNA reduction up to 84% ; significant protein level reduction |
| Functional Effects | MTT assays, flow cytometry, colony formation | Inhibited proliferation and colony formation; cell cycle arrest |
| Transduction Efficiency | Fluorescence microscopy, flow cytometry | Exceeding 70% in various cell types |
| Biological Reproducibility | Statistical analysis of replicates | P<0.05 significance in functional assays |
Features of Gene Silencing
- Industrial Scalability: The growing demand for LV-based cell therapies, particularly CAR-T, is driving the industry toward robust, scalable, and cost-effective manufacturing.
- Targeted Delivery: Next-generation LVs will enhance cell-specific targeting through envelope engineering (e.g., ligand retargeting pseudotyping), minimizing off-target effects and systemic toxicity for in vivo applications.
- CRISPR/Cas9 Integration: LVs are increasingly being used to deliver the components of the CRISPR/Cas9 system—Cas9 nuclease and guide RNA (gRNA) or essential trans-acting elements—to achieve more durable gene knockout or targeted gene correction, a powerful evolution of the transient nature of RNAi.
What Makes Creative Biolabs Your Top Choice
Unparalleled Expertise
Our team of doctoral level scientists provides active consultation on all aspects of carrier design, from promoter selection to orientation optimization.
Safety First Concept
We only use the third-generation SIN system, which has strict RCL and quality testing, and prioritize biosafety for research and preclinical use.
Guaranteed Performance
We provide high titer production and knockout efficiency guarantees for our customized shRNA lentivirus services.
Wide Range of Applications
Our carriers are optimized for the most demanding applications, including central nervous system gene transfer, CAR T cell engineering, and stable transduction of various primary and stem cells.
Frequently Asked Questions
Q1: What is the typical gene silencing efficiency achieved using lentiviral vectors?
A: Our system typically achieves 70–90% reduction in target mRNA levels, as verified by qRT-PCR in standard cell lines. Actual efficiency may vary depending on target accessibility, cell type transduction efficiency, and shRNA design. We offer multiple shRNA sequences for each target to ensure at least one achieves high efficacy and include optimization guidance for challenging targets.
Q2: How long does the silencing effect last after transduction?
A: With standard integrating vectors, the silencing effect typically persists throughout the lifespan of the cell population because the shRNA expression cassette is stably integrated into the host genome. In dividing cells, we have observed measurable silencing for at least 4–8 weeks without selection. With IDLVs, the effect gradually diminishes within 1–3 weeks, depending on the cell proliferation rate.
Q3: What safety features are included in your lentiviral vectors?
A: All of our vectors include a third-generation SIN configuration with a deletion of the enhancer/promoter region in the LTR. We also offer optional additional safety features, including tissue-specific promoters, miRNA-mediated detargeting, and an integration-deficient option using a D64 mutation in the integrase gene. These features minimize the risks associated with insertional mutagenesis and off-target expression.
Q4: Can your vectors transduce difficult-to-transfect cells, such as primary neurons or stem cells?
A: Yes, our advanced pseudotyping options significantly improve transduction efficiency in challenging cell types, including primary neurons, hematopoietic stem cells, and quiescent populations. We offer specialized envelopes, such as Mokola-G and Rabies-G, and custom-engineered glycoproteins, to improve transduction of difficult-to-transduce cell types. Additionally, we offer transduction enhancers that can increase efficiency in challenging cells by 3-5 times.
Q5: What is the typical timeline for a custom lentiviral vector project?
A: A standard project typically takes 4-6 weeks, from sequence confirmation to viral preparation delivery. This includes vector construction, viral packaging, titer determination, and quality control. Expedited services are available to expedite this timeline, with pre-made vectors for common targets available for immediate shipment.
Reach Out to Us Now!
Creative Biolabs is a leading commercial supplier of viral vector technologies, offering a full range of high-quality custom services for LVs design, including LVs design for targeting stem cell research, cellular reprogramming, immune modulation, gene editing. Our LVs can be used for gene function research, preclinical target validation, gene therapy & vaccine development for academic use or industrial R&D. Please feel free to contact us for more information.
Reference
- Koenig O, Walker T, Perle N, et al. New aspects of gene-silencing for the treatment of cardiovascular diseases. Pharmaceuticals, 2013, 6(7): 881-914. https://doi.org/10.3390/ph6070881 (Distributed under Open Access license CC BY 4.0, without modification.)
