Optimization of Bicistronic Lentiviral Vector Service
Bicistronic lentiviral vector is a type of newly developed therapeutic gene delivery system with the ability of co-expressing two genes in infected cells. As an undisputed gene therapy service provider, Creative Biolabs offers a full range of bicistronic lentiviral vector construction services based on our powerful GTOnco™ Platform and abundant experience.
Introduction to Bicistronic Lentiviral Vectors
Generally, a bicistron is termed as two mRNA molecules encoding different peptide chains that are linked together by a short sequence of non-coding proteins. Actually, the DNA fragments encoding these two peptide chains are located in the same transcription unit and share the same starting point and endpoint, which means that the two peptide chains are usually expressed simultaneously. Exactly, the property that co-expression of two or more (multicistron) specific genes can be promisingly applied for many biological fields, especially for gene therapy.
Lentiviral vectors are useful tools for delivering the target genes into the body or modifying the genes in both dividing and non-dividing cells using lentiviruses. The transgene capacity of lentiviral vectors is much larger than common vectors, which makes those lentiviruses the most effective vehicles to transfer large or complex genes, such as bicistron (or multicistron) to cells. Moreover, bicistronic lentiviral vectors technology allows to simultaneously deliver and co-express two proteins in any cell type.
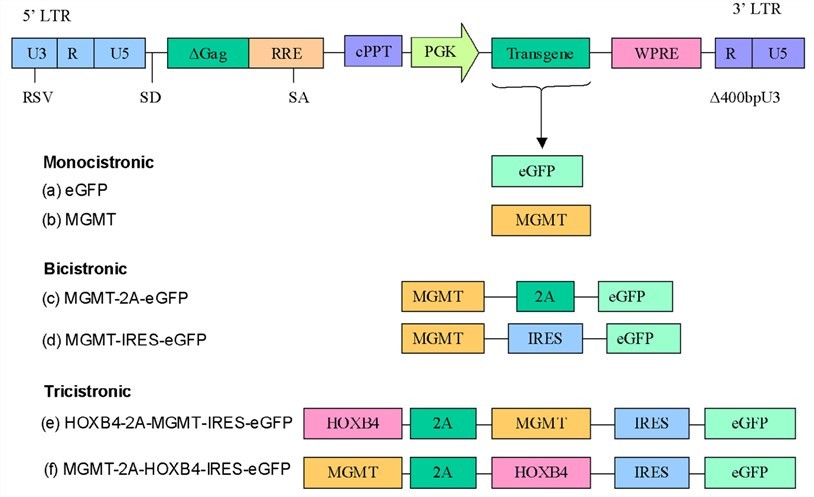 Figure 1. Schematic diagram of HIV-1 based lentiviral vectors.1
Figure 1. Schematic diagram of HIV-1 based lentiviral vectors.1
Common Bicistronic Expression Strategies
Lentiviral vectors (LVs) are powerful tools capable of integrating large genetic payloads into the genome of dividing and non-dividing cells. While monocistronic vectors express a single gene, bicistronic systems utilize specialized genetic elements to drive the transcription and translation of two open reading frames (ORFs) from a single construct.
IRES-based Vectors
- Mechanism: An Internal Ribosome Entry Site (IRES) is placed between Gene A and Gene B. The ribosome translates Gene A via the 5' Cap and then re-initiates translation at the IRES for Gene B.
- Pros: No modification to the protein sequence.
- Cons: Gene B is typically expressed at significantly lower levels (10–50% of Gene A) due to lower translation initiation efficiency.
2A Peptide-mediated Co-expression
- Mechanism: Viral 2A peptides cause "ribosomal skipping" during translation. This results in the production of two separate proteins from a single mRNA transcript.
- Pros: Near-equimolar (1:1) expression of both genes; compact size.
- Cons: Adds a small peptide residue to the C-terminus of the upstream protein and a proline to the N-terminus of the downstream protein.
Dual-Promoter Systems
- Mechanism: Two separate promoters drive two distinct expression cassettes within the same vector.
- Pros: Independent control of gene expression levels.
- Cons: Prone to Promoter Interference (transcriptional suppression of the downstream promoter) and reduced viral titer due to increased vector size.
Applications of Optimized Bicistronic Lentiviral Vectors
- CAR-T/CAR-NK Cell Engineering: Co-expression of the Chimeric Antigen Receptor (CAR) and a survival cytokine (e.g., IL-15) or a safety switch.
- Reprogramming: Co-expression of transcription factors for iPSC generation.
- Functional Genomics: Co-expression of a gene of interest and a fluorescent reporter for cell sorting and tracking.
- Antibody Production: Simultaneous expression of Heavy Chain and Light Chain for recombinant antibody generation.
Services At Creative Biolabs
Custom Bicistronic Vector Design & Construction
We build your vector from the ground up, ensuring the genetic architecture is optimized for stability and expression before wet-lab work even begins.
- Codon Optimization: Proprietary algorithms to optimize both Gene A and Gene B for human (or murine) codon usage, removing rare codons and cryptic splice sites.
- Backbone Engineering: Selection of 3rd generation SIN backbones with optimized cis-acting elements (cPPT, WPRE) to maximize nuclear import and transcript stability.
- Cloning Strategy: Precise insertion of your genes of interest (GOIs) into IRES-based or 2A-peptide-based scaffolds.
Dual-Promoter Vector Engineering
For projects requiring independent regulation of two genes, we resolve the common issue of transcriptional interference.
- Interference Analysis: Testing different promoter orientations (Convergent vs. Divergent) to minimize read-through.
- Promoter Pairing: Screening compatible promoter combinations to ensure the strong upstream promoter does not silence the downstream cassette.
Insulator Insertion: Adding chromatin insulators (e.g., cHS4) to separate the expression cassettes and prevent silencing.
High-Titer Bicistronic Lentivirus Packaging
Bicistronic vectors are often large and difficult to package. Our optimized production platform overcomes these physical limitations.
- Pilot Scale Packaging: Small-scale production for initial clone screening.
- Large Scale / In Vivo Grade: Up to 50 mL of ultra-purified virus. We utilize optimized plasmid ratios specifically tuned for large genomes to ensure high functional titers.
Our One-Stop Optimization Strategy
We provide a comprehensive Optimization of Bicistronic Lentiviral Vector Service. Unlike off-the-shelf solutions, our service employs a data-driven approach to fine-tune every component of the vector—from the choice of linker (IRES vs. 2A peptides) and promoter configuration to the backbone architecture. We resolve common issues such as transcriptional interference and low viral titers, ensuring that your bicistronic vector delivers optimal performance for CAR-T development, stem cell engineering, and in vivo gene therapy.
Advantages of Our Services
In terms of co-expressing your genes of interest, Creative Biolabs is a first-rate biotech company who has comprehensive development capability to provide you a wide range of customized gene therapy services. We have absolute advantages in offering lentiviral vector services:
- Customized design of bicistron (or multicistron) based on clients' demands
- High-quality lentiviral vectors construction
- Various fluorescence labels are available
- High-efficiency expression of your genes of interest
Our Collaborative Process
At the core of our service is a partnership model designed for transparency, efficiency, and success. We believe that the best outcomes are achieved through close collaboration and iterative feedback. Our process is structured to keep you informed and in control at every stage.
-
Phase 1
In-Depth Discovery & Strategic Planning
- Initial Consultation: We begin with a comprehensive discussion to understand your scientific objectives, target cells, desired expression levels, and final application (e.g., in vitro research, in vivo study, cell therapy development).
- Feasibility & Strategy Proposal: Our experts analyze your gene sequences and requirements. We then present a detailed project plan outlining 2-3 tailored optimization strategies, a clear timeline, and a transparent quotation.
- Joint Strategy Finalization: We review the proposal together, incorporating your feedback to finalize the experimental roadmap before any work begins.
-
Phase 2
Co-Development & Iterative Design
Vector Design & Cloning: Our molecular biology team executes the agreed-upon design. You receive annotated vector maps for review prior to synthesis or cloning.
Preliminary Data Sharing & Review: We provide early data, such as plasmid sequencing confirmations and initial small-scale transfection results (e.g., expression checks in HEK293T cells). This is a key checkpoint.
Iterative Feedback Loop: Based on your review of the preliminary data, we can make adjustments before proceeding to large-scale viral production. This agile approach saves time and resources.
-
Phase 3
Execution, Validation & Joint Analysis
- Virus Production & QC: We proceed to optimized lentiviral packaging, purification, and rigorous quality control (physical and functional titering).
- Comprehensive Functional Validation: We perform the agreed-upon assays in relevant cell lines. Raw data and preliminary analyses are shared with you.
- Data Analysis Meeting: We schedule a dedicated session to walk you through all validation results—from flow cytometry histograms to Western blot images—and discuss their interpretation in the context of your project goals.
-
Phase 4
Delivery, Support & Knowledge Transfer
- Final Delivery Package: You receive the complete deliverables: optimized plasmids, high-titer virus (if selected), a comprehensive data package, and a detailed final report.
- Project Handover & Support: We provide a clear handover, including recommended storage conditions and handling protocols. Our team remains available for ongoing technical support to ensure your downstream experiments are successful.
- Long-Term Partnership: We value becoming your trusted partner for future vector needs, building on the insights and optimized platforms developed during our collaboration.
Frequently Asked Questions
Q: IRES vs. 2A Peptide: Which Is Better for My Project?
A: IRES results in weaker downstream gene expression, but the two proteins are completely independent; 2A peptide provides more balanced expression, but leaves a small number of residues at the C-terminus and N-terminus. If the downstream gene requires low expression levels or the protein is sensitive to terminal modifications, IRES is a suitable option; if a 1:1 stoichiometric ratio is required and a small amount of peptide residue is tolerable, 2A is usually a more efficient choice. We will help you determine the best solution through preliminary experiments.
Q: How to Achieve Balanced Expression of Two Genes?
A: Balanced expression requires systemic optimization, including testing gene order, screening different 2A peptide or IRES variants, adjusting UTR sequences, and even employing strategies to weaken the translation intensity of the upstream gene. There is no universal solution; we provide customized optimization pathways for you.
Q: What Is the Maximum Insert Size Supported?
A: The packaging limit for lentiviral vectors is approximately 9-10 kb. For bicistronic vectors, the total insert size (including promoter, genes, and regulatory elements) is usually recommended to be kept within 6 kb to ensure high-titer virus production. We will evaluate your sequence and provide suggestions for optimization.
Q: How do you solve the "Promoter Interference" in dual-promoter vectors?
A: We use transcript termination signals (polyA) between cassettes or place the promoters in specific orientations (e.g., divergent or convergent) to minimize read-through and steric hindrance.
Connect with Us Anytime!
At Creative Biolabs, we understand that achieving perfect co-expression is not a "one-size-fits-all" task. We have modularized our expertise into specific service units, allowing you to choose the exact level of optimization your project requires—from design to large-scale viral production. For more detailed information, please feel free to contact us or directly send us an online inquiry.
Reference
- Chinnasamy, D.; et al. (2006). Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virology Journal. 3:14. 10.1186/1743-422X-3-14 (Distributed under Open Access license CC BY 4.0, without modification.)
