Tissue-specific Promoter-regulated Lentiviral Vectors Service
Lentiviral vectors, as efficient gene delivery tools, have achieved significant progress in human gene therapy. Armed with powerful technologies and professional scientists, Creative Biolabs has successfully established an advanced gene therapy platform to provide customized one-stop tissue-specific promoter-regulated lentiviral vectors construction services to global clients.
Lentiviral Vectors Introduction
In recent years, lentiviral vectors have been the focus of gene therapy development owing to their outstanding advantages, such as (i) capable to infect both dividing cells and non-dividing cells, even difficult-to-infect cells and terminally differentiated cells; (ii) high efficiency of gene transfection and long-term gene expression; (iii) ability to deliver larger gene fragments and cDNA, etc. Due to these advantages, lentiviral vectors-based gene therapy has been widely explored and made great success in the past several years, covering from the treatment of cancers, diabetes, autoimmune disease to vascular transplants.
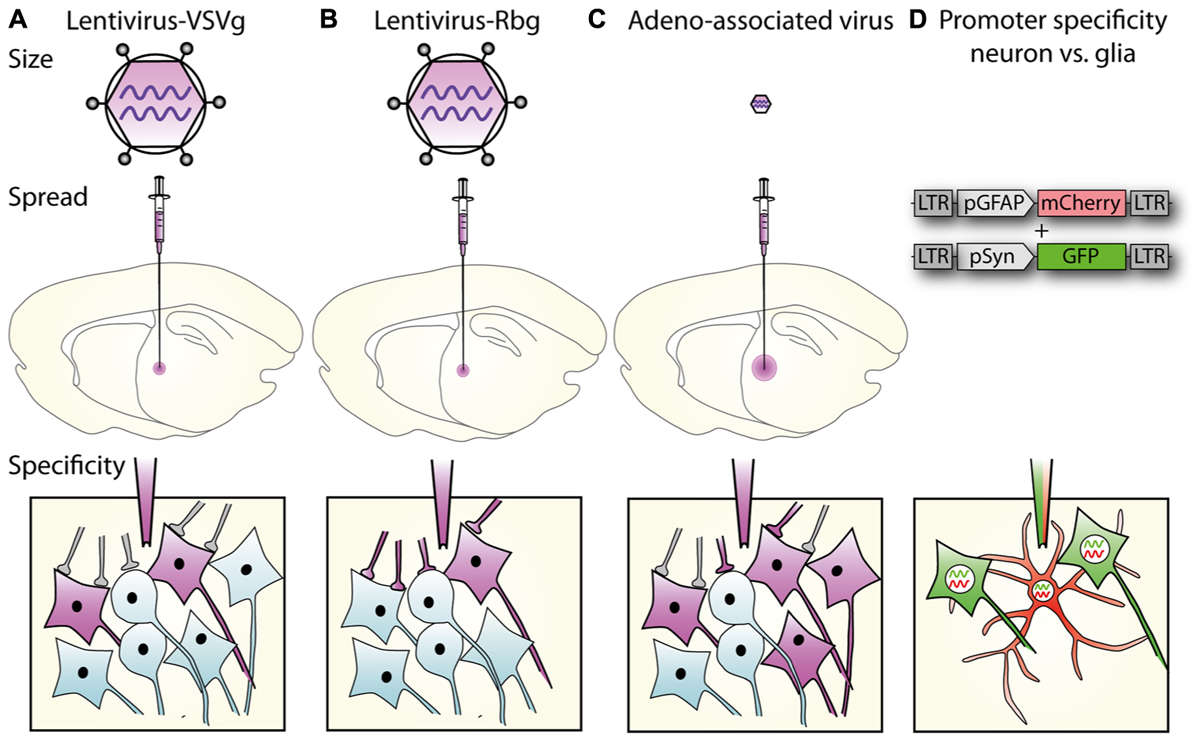 Figure 1. Lentiviral vector spread, transduction, and expression is mediated by particle size, envelope properties and promoter usage.1
Figure 1. Lentiviral vector spread, transduction, and expression is mediated by particle size, envelope properties and promoter usage.1
Scientific Rationale of Tissue-specific Promoters
The specificity of lentiviral vectors is driven by the precise interaction between cis-acting regulatory elements (promoters/enhancers) and the unique trans-acting factors (transcription factors) present within the target cell's nuclear environment.
- Regulatory Mechanism: Tissue-specific promoters utilize specific binding motifs that are only recognized by transcription factors expressed in the target lineage.
- Specificity vs. Strength: Native tissue-specific promoters often exhibit lower activity than viral promoters. We overcome this by optimizing enhancer elements to strike a balance between high specificity and robust expression levels.
- Chromatin Environment: To mitigate the risk of promoter silencing or positional effects upon genomic integration, our vectors can be engineered with insulator elements (e.g., cHS4) to maintain an open chromatin state for long-term expression.
Comparison with AAV Tissue-Specific Strategies
Lentivirus advantages include larger capacity and integration properties, making them particularly suitable for applications requiring long-term stable expression of larger genes. AAV is often preferred for clinical gene therapy. The choice depends on specific needs, and the two systems can be complementary.
| Aspect | Lentivirus | AAV |
|---|---|---|
| Capacity | Larger (~8 kb) | Smaller (~4.7 kb) |
| Integration | Genomic integration, long-term expression | Mainly episomal, long-lasting but may be lost |
| Immunogenicity | Moderate | Lower, but pre-existing neutralizing antibodies exist |
| Tissue Specificity Strategy | Primarily relies on promoter | Promoter + serotype tropism |
| Application Scenarios | Suitable for in vitro and in vivo, preferred when integration is needed | Commonly used for in vivo gene therapy |
Tissue-specific Promoter-regulated Lentiviral Vectors Construction
A promoter is a DNA region located upstream towards the 5' end of the gene. The key factors for tissue-specific promoter-regulated lentiviral vectors construction are designation and introduction of a specific promoter since the promoter contains conserved sequences required for RNA polymerase and transcription factors binding. The promoter initiates the mRNA transcription when it is recognized and bound by RNA polymerase. Meanwhile, it contains a binding site for transcription factors, whose responsibility is recruiting the specific RNA polymerase and regulating gene expression in specific cells and time. Therefore, the introduction of different promoters will result in lentiviral vectors with different targeting specificity.
Until now, many kinds of tissue-specific promoters have been discovered and identified, such as albumin promoter in the liver, muscle creatinine kinase promoter in muscle tissue, myelin basic protein promoter in glial cells and oligodendrocytes. Furthermore, several tumor tissue/cell selective promoters also have been isolated, such as α-fetoprotein in the liver tumor, α-lactalbumin and β-lactoglobulin promoters in the breast cancer, L-plastin promoter in the cancer cells, etc.
Comparative Advantages Over Ubiquitous Promoters (CMV / EF1α / PGK)
| Feature | Ubiquitous Promoters | Tissue-Specific Promoters |
|---|---|---|
| Expression Breadth | Broad, multiple cell types | Restricted to specific tissues/cells |
| Safety | Potential off-target risks | High targeting specificity, improved safety |
| Expression Strength | Typically high | Moderate, but sufficient in target cells |
| Application Scenarios | Basic overexpression, screening | Targeted therapy, precise functional studies |
Services At Creative Biolabs
Creative Biolabs is an expert in terms of gene therapy after years of effort and progress. Our Ph.D. scientists and professional staff are proud to offer targeting modification services to improve the performance of lentiviral vectors for both basic research and clinical applications. Our featured lentiviral vectors services mainly include but not limited to:
- Tissue-specific promoters' custom design
- Lentiviral vectors design and preparation
- Construction of promoter-regulated lentiviral vectors
- Detection and verification of optimized lentiviral vectors
- Safety evaluation
Lentivirus Production & Quality Control
We adhere to strict SOPs to ensure high-titer, clinical-grade ready research vectors.
Production: Transfection of HEK293T cells followed by ultracentrifugation and chromatography purification.
Quality Control (QC):
- Physical Titer: qPCR (Genomic copies/mL).
- Functional Titer: Flow cytometry (Transducing Units, TU/mL).
- Sterility: Bacteria and Fungi negative.
- Mycoplasma: Negative.
- Endotoxin: < 10 EU/mL (for in vivo grades).
- RCL Testing: Replication-Competent Lentivirus negative.
Validation & Functional Assays
To ensure the vector works as intended, we offer validation services:
- In Vitro Validation: Transduction of relevant cell lines vs. control lines.
- Expression Analysis: qPCR, Western Blot, and Immunofluorescence (IF).
- Reporter Assays: GFP/Luciferase imaging to verify specificity ratios.
- In Vivo Profiling: Systemic injection in mice followed by organ harvesting and biodistribution analysis.
Applications of Our Services
- Targeted Gene Therapy: Developing safer vectors for genetic disorders.
- Animal Model Construction: Creating tissue-specific transgenic mice/rats via lentiviral injection.
- Functional Screening: CRISPR screening in specific cell populations within heterogeneous tissues.
- Lineage Tracing: Long-term labeling of stem cells and their progeny in vivo.
Advantages of Our Service
Extensively Validated Tissue-Specific Promoter Library
- Covers major tissues and cell types
- Rigorously validated in vitro and in vivo, ensuring reliable specificity
- Continuously updated and optimized promoter collection
High Titer, Low Background Expression
- Optimized packaging system for high viral yield
- Promoter design minimizes leaky expression
- High-transduction-efficiency virus preparations
Flexible Customization Capabilities
- End-to-end customization from promoter selection to vector design
- Accommodation of complex requirements (multi-gene, inducible, miRNA regulation, etc.)
- Rapid construction and iteration capabilities
Stringent Quality Control System
- Comprehensive quality control checkpoints throughout the process
- Full safety testing (RCL, endotoxin, etc.)
- Detailed QC reports and data support
Project Workflow
-
Phase 1
Requirement Assessment & Protocol Design
- Client consultation and requirement communication
- Promoter selection and vector design recommendations
- Protocol confirmation and quotation
-
Phase 2
Promoter & Vector Construction
- Promoter cloning or synthesis
- Vector construction and clone verification
- Plasmid preparation and QC
-
Phase 3
Lentivirus Packaging & Purification
- Virus packaging and production
- Concentration and purification
- Buffer exchange and aliquoting
-
Phase 4
Quality Testing
- Titer determination
- Safety and purity testing
- Preliminary specificity validation (if selected)
-
Phase 5
Delivery & Technical Support (Ongoing)
- Delivery of virus preparations and documents
- Usage guidance and technical support
- Follow-up experiment consultation
Frequently Asked Questions
Q1: How do I select the right tissue-specific promoter?
A: Selection depends on the target cell type and the required expression level. We recommend reviewing literature for markers highly expressed in your target cells. Our team can also assist in selecting or designing hybrid promoters.
Q2: Can these vectors maintain expression long-term in vivo?
A: Yes. Lentiviral vectors integrate into the host genome. However, promoter silencing can occur over time. We can include chromatin insulators to minimize silencing and ensure stable long-term expression.
Q3: Does the promoter affect the viral titer?
A: The internal promoter usually does not affect the packaging titer, as the viral RNA is driven by the promoter in the LTR (or RSV/CMV in the packaging plasmid) during production. However, the internal promoter determines the expression level after transduction.
Q4: How does this compare to AAV tissue specificity?
A: AAV achieves specificity through both capsid tropism and promoters, but has a limited packing capacity (~4.7kb) and remains largely episomal. Lentivirus has a larger capacity (~8-10kb), integrates for permanent expression, and relies almost exclusively on the promoter for specificity.
Connect with Us Anytime!
Creative Biolabs provides a specialized Tissue-specific Promoter-regulated Lentiviral Vector Service. By combining the broad tropism and stable integration capabilities of lentiviral vectors with the precise regulatory control of tissue-specific promoters, we enable researchers to target gene expression exclusively to specific organs (e.g., liver, brain, muscle) or distinct cell lineages. This service is essential for refining gene therapy strategies, creating physiologically relevant disease models, and conducting precise functional screens. If you are working on lentiviral vector-based gene therapy or interested in the optimization of lentiviral vectors, you can directly contact us or communicate with us for more detailed information.
Reference
- Parr-Brownlie L C, Bosch-Bouju C, Schoderboeck L, et al. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Frontiers in molecular neuroscience, 2015, 8: 14. https://doi.org/10.3389/fnmol.2015.00014 (Distributed under Open Access license CC BY 4.0, without modification.)
