Recombinant Adenovirus Construction Service in Bacterial Systems
The development of recombinant adenoviruses begins not in mammalian cells, but in the highly efficient and scalable environment of bacterial systems. The construction of a genetically precise and stable adenoviral genome within a bacterial plasmid represents the critical first step in this process, forming the foundational blueprint from which all subsequent viral production flows. This stage demands unparalleled accuracy in molecular biology, as even minor errors in the vector can be amplified catastrophically during later viral rescue and amplification.
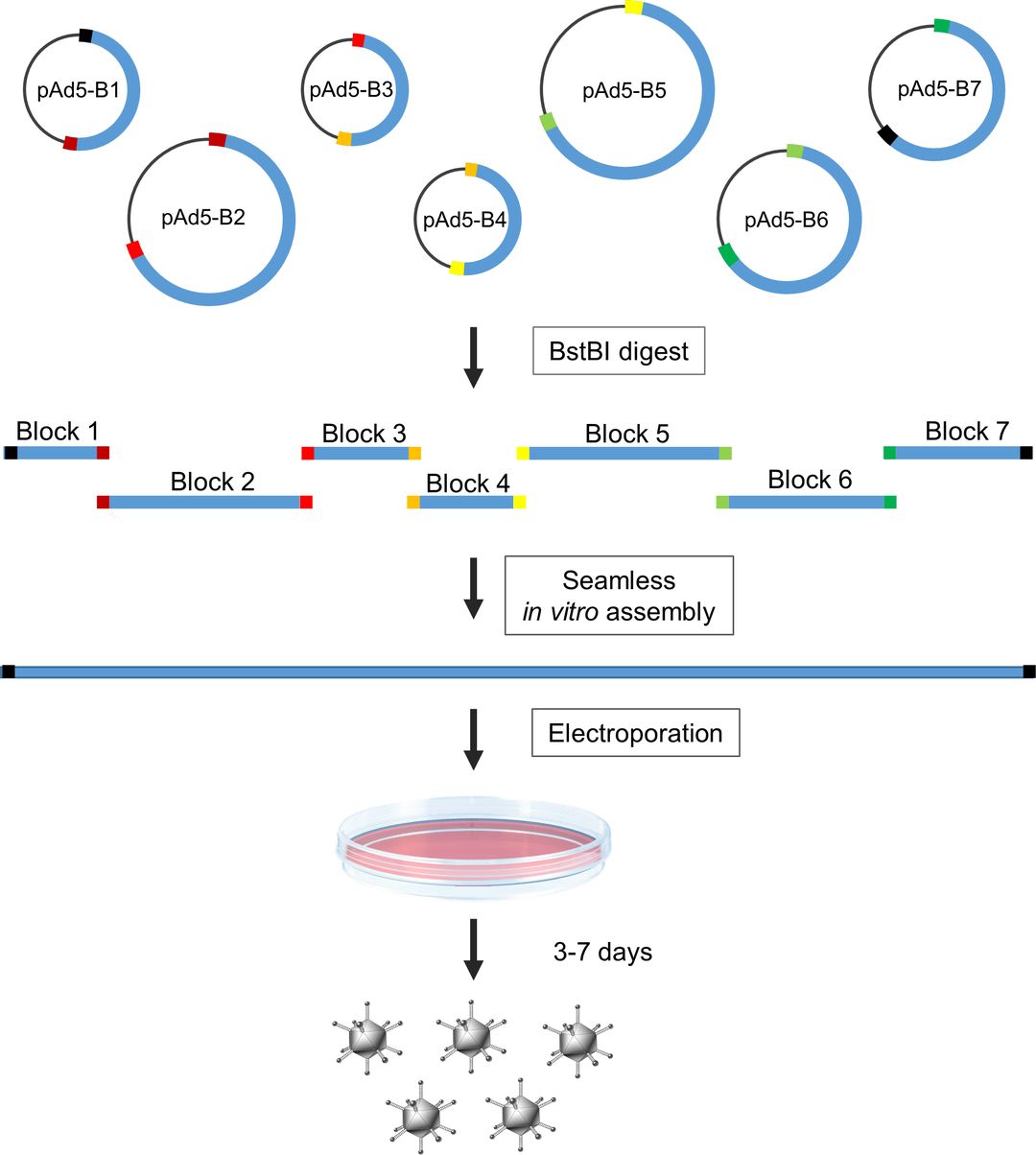 Fig.1 Seamless assembly of recombinant adenovirus genomes in high-copy bacterial plasmids 1,2
Fig.1 Seamless assembly of recombinant adenovirus genomes in high-copy bacterial plasmids 1,2
Our Recombinant Adenovirus Construction Service in Bacterial Systems is specifically engineered to deliver this foundational precision. We specialize in the sophisticated molecular engineering required to seamlessly clone your gene of interest into a validated adenoviral backbone, providing you with a sequence-guaranteed, high-yield plasmid ready for transfection into packaging cells. By mastering this initial, complex phase, we empower your research with a flawless starting point, ensuring efficiency and success in all downstream applications.
Our Streamlined Construction Workflow
Our process is a model of molecular efficiency, leveraging the power of bacterial homologous recombination to transform your genetic design into a physical, high-quality plasmid construct.
Project Scoping & Design Analysis: We begin with a detailed consultation to analyze your gene of interest and experimental goals. Our experts advise on optimal plasmid backbone selection (e.g., AdEasy, Adeno-X), promoter choice, and cloning strategy to ensure optimal performance.
Preparation of Genetic Components: Based on the design, we prepare the adenoviral backbone plasmid (e.g., pAdEasy-1) and generate your linearized shuttle vector or PCR-amplified transgene cassette with the necessary homologous arms for recombination.
Transformation & Homologous Recombination: The critical step involves co-transforming the linearized backbone and your transgene fragment into specialized, high-efficiency E. coli cells (e.g., BJ5183-AD-1). These cells facilitate the precise homologous recombination event that assembles the complete, circular recombinant adenoviral plasmid.
Clonal Selection & Plasmid Amplification: Transformed cells are plated for single-colony isolation. Positive clones are selected based on antibiotic resistance and/or LacZ alpha-complementation screening. A single, positive colony is then expanded in a liquid culture to amplify the recombinant plasmid.
Rigorous Sequence Verification: The amplified plasmid is purified, and the entire cloned expression cassette—including promoter, gene of interest, polyA signal, and flanking ITR regions—is subjected to 100% Sanger sequencing. This non-negotiable step confirms the absolute fidelity and integrity of the construct.
Large-Scale Plasmid Production: Following sequence confirmation, the plasmid is transformed into a standard, high-copy-number E. coli strain (e.g., DH5α, Stbl3) for large-scale fermentation, yielding milligram quantities of high-purity, endotoxin-free plasmid DNA.
Final QC & Delivery: The bulk plasmid is subjected to a final quality control check, including restriction enzyme digestion to verify the expected pattern. You receive a substantial aliquot of the purified plasmid, a comprehensive sequence alignment report, and a detailed data sheet.
Core Service Highlights
| Service Phase | Key Activities & Distinctive Value |
|---|---|
| Vector Strategy & Design | Expert consultation on backbone selection, promoter compatibility, and multi-gene arrangements to maximize experimental success. |
| Advanced Cloning | Utilization of proven homologous recombination systems in specialized bacterial cells for seamless, high-efficiency assembly. |
| Quality Assurance | 100% Comprehensive Sequencing of the entire expression cassette and critical ITRs to guarantee genetic accuracy and functionality. |
| Scalable Production | Large-scale plasmid preparation in optimized strains, providing ample high-quality DNA for immediate use and long-term storage. |
Our Distinct Advantages
Unrivaled Expertise in Homologous Recombination: We have perfected the use of bacterial homologous recombination systems, which offer significant advantages over traditional restriction-ligation cloning. This method allows for the seamless assembly of large fragments without reliance on internal restriction sites, enabling more flexible and sophisticated vector designs with higher success rates for complex constructs.
Guaranteed Sequence Fidelity: We consider full-sequence verification a mandatory deliverable, not an optional extra. By sequencing the entire expression cassette and the fragile ITR regions—critical for viral DNA replication—we provide irrefutable proof of your plasmid's integrity, eliminating a major source of experimental failure and saving you invaluable time and resources.
Optimized for Downstream Success: Our constructs are not just molecular clones; they are engineered for high-efficiency rescue in mammalian cells. We use validated, production-proven adenoviral backbones that minimize the generation of replication-competent adenoviruses (RCA) and are optimized for high-titer virus production after transfection into packaging cell lines.
Flexible and Customized Cloning: Our platform is adaptable to your specific needs. We can start from your provided plasmid, a synthesized gene fragment, or even a complex multi-gene cassette. This flexibility allows us to accommodate a wide range of project requirements, from simple gene overexpression to the construction of intricate, tissue-specific, or inducible expression systems.
Speed and Cost-Efficiency: By leveraging the rapid growth and scalability of bacterial systems, we deliver a flawless recombinant adenoviral plasmid in a fraction of the time and cost it would take to attempt viral rescue directly from a non-optimized construct. This accelerates your entire research timeline, getting you to functional virus and meaningful data faster.
What Our Clients Say?
"We needed to construct a dual-promoter adenovirus for cell-type specific expression, a project that had stalled with our in-house capabilities. The team's mastery of homologous recombination was the key. They delivered a perfect plasmid on the first attempt, and the sequencing report was so comprehensive it gave us complete confidence to proceed directly to virus production. This service turned a bottleneck into a breakthrough."
— Dr. Alex Thompson, Principal Investigator
"As a CDMO, we require partners who deliver absolute precision and documentation. Their adenovirus construction service provided both. The plasmid was flawless, the yield was exceptional, and the accompanying documentation, including the full sequence alignment, was exactly what we need for our regulatory filings. They have become our trusted partner for all our foundational vector construction."
— Sarah Mitchell, Director of Process Development
"Our goal was to create a library of adenoviruses for a high-throughput CRISPR screen. The scalability and reliability of their bacterial construction service were critical. They managed the entire process seamlessly, delivering a large set of sequence-verified plasmids on time. The consistency across the entire library has been outstanding, which is fundamental for the quality of our screen."
— Dr. Kevin Zhao, Senior Scientist
FAQ
Q: Why is bacterial system construction a prerequisite for adenovirus production?
A: Mammalian cells are excellent for virus assembly and production but are inefficient and unreliable for the complex molecular engineering required to build large, precise DNA constructs. Bacterial systems, with their rapid division, high transformation efficiency, and powerful innate homologous recombination machinery, provide the ideal environment for the accurate and scalable construction of the large (~35 kb) adenoviral plasmid, which is then simply "plugged into" the mammalian system for virus production.
Q: What is the key advantage of using homologous recombination over traditional cloning?
A: Traditional cloning is limited by the availability and positioning of restriction enzyme sites. Homologous recombination is a seamless, site-independent method that uses short homologous sequences (arms) to direct the assembly. This is far more efficient for large fragments, allows for easier cloning of complex cassettes, and avoids the introduction of unwanted linker sequences, preserving the native function of your genetic elements.
Q: What starting materials do I need to provide?
A: We offer maximum flexibility. You can provide a plasmid containing your gene of interest, from which we can amplify the fragment. Alternatively, you can simply provide us with the DNA sequence, and we can handle the entire process from de novo gene synthesis to final assembly and verification.
Q: How do you ensure the stability of the ITRs in the bacterial system?
A: The Inverted Terminal Repeats (ITRs) are structurally unstable in standard E. coli strains. We address this by using specialized, recombination-deficient bacterial strains (e.g., Stbl3) for the final plasmid amplification and large-scale production. These strains suppress rearrangements, ensuring the ITRs—which are essential for viral genome replication—remain intact and functional in the final plasmid product.
Q: What is the final deliverable I will receive?
A: Upon completion, you will receive a substantial quantity of the high-purity, endotoxin-free recombinant adenoviral plasmid DNA, ready for transfection. This is accompanied by a comprehensive report including gel electrophoresis data, restriction digest analysis, and the critical 100% sequence verification report for the entire cloned region.
Conclusion
The path to a successful recombinant adenovirus experiment is built upon the quality of its DNA blueprint. A poorly constructed plasmid leads to failed rescues, low viral titers, and confounding experimental results. Our Recombinant Adenovirus Construction Service in Bacterial Systems eliminates this primary risk factor. By delivering a molecularly perfect, sequence-verified plasmid through our optimized homologous recombination platform, we provide the most reliable and efficient starting point for your viral vector projects. Partner with us to lay a foundation of uncompromising quality, ensuring that your investment in downstream rescue, production, and experimentation yields the robust, reproducible, and meaningful data your research demands.
References
- Miciak JJ, Hirshberg J, Bunz F (2018) "Seamless assembly of recombinant adenoviral genomes from high-copy plasmids" PLOS ONE 13(6): e0199563. https://doi.org/10.1371/journal.pone.0199563
- Distributed under Open Access license CC BY 4.0, without modification.
