Adenoviral Vector Design Service for RNAi Delivery
Adenoviral Vectors for RNAi Delivery
Adenoviral vectors have emerged as a pivotal tool in human gene therapy, boasting high transduction efficiency across a broad spectrum of target tissues and a favorable safety profile validated in both animal models and human clinical trials. Meanwhile, RNA interference (RNAi) has also established itself as a crucial asset in this field, enabling scientists to shift from classical gene transfer strategies to gene silencing approaches—or even combinations of the two. However, a key challenge in achieving effective RNAi lies in delivering it to the intended organs and target cells. Notably, adenoviral vector-based RNAi technology has already been successfully applied in various cell types and tissues, addressing this delivery hurdle to a significant extent.
Specifically, RNAi functions as a natural gene-silencing mechanism conserved in plant and mammalian cells, encompassing both initiation and effector steps. The initiation phase is triggered by double-stranded RNA (dsRNA), which is cleaved by the ribonuclease III-type enzyme Dicer (Dcr) into small interfering RNAs (siRNAs)—short 21-23 nt duplexes with a symmetric 2 nt overhang at the 3'-end, a 5'-phosphate group, and a 3'-hydroxy group. These siRNAs then integrate into a nuclease-containing multi-protein complex known as the RNA-induced silencing complex (RISC). Activation of RISC occurs via RNA helicase activity, which leads to the dissociation of one strand of the siRNA duplex. In the effector phase, the remaining single-stranded siRNA guides post-transcriptional gene silencing: this is achieved either through the degradation of target mRNAs or the induction of translational inhibition via the miRNA pathway.
For RNAi delivery, most adenoviral vectors are derived from human serotypes 2 and 5—two of the most commonly used adenoviruses in gene therapy. In terms of delivery strategies, the H1-RNA promoter typically drives siRNA expression, while the RNA polymerase II (RNA pol II) CMV promoter has been shown to mediate effective gene silencing both in vitro and in vivo. Adenoviral vectors engineered to express siRNA or shRNA (short hairpin RNA) have proven highly effective in specific cell types, including islet β-cells, cardiovascular cells, and lung cancer cell lines. Furthermore, regulated adenoviral vectors offer additional advantages: they not only can infect a wide range of replicating and non-replicating cells but also enable temporal control over gene silencing.
| Comparison Dimensions | Adenovirus (AdV) | Lentivirus (LV) | Adeno-Associated Virus (AAV) |
|---|---|---|---|
| Infection Efficiency | Advantages: Highly efficient in both dividing and non-dividing cells; broad host range; rapid delivery in vitro and in vivo | Advantages: Can infect both dividing and non-dividing cells, especially suitable for hard-to-transfect cells like primary cells and hematopoietic stem cells | Advantages: Extremely broad host range, covering almost all cell types; specific serotypes (e.g., AAV9) can target muscle/nerve tissues |
| Disadvantages: Dependent on CAR receptors; low infection efficiency in immune cells such as dendritic cells | Disadvantages: Slightly lower infection efficiency in some epithelial cells than AdV; dependent on receptors such as CD4/CXCR4 | Disadvantages: Low infection efficiency in some tumor cells; pre-existing antibodies may reduce efficiency | |
| Immunogenicity | Advantages: None | Advantages: Low immunogenicity; envelope derived from host cells, not easily recognized by the immune system | Advantages: Extremely low immunogenicity; no autonomous replication ability; does not express viral proteins; allows repeated injections |
| Disadvantages: Strong immunogenicity; capsid proteins trigger humoral/cellular immunity (e.g., activation of NK cells and T cells); easily cleared; may cause inflammation; difficult for repeated use | Disadvantages: Residual packaging cell proteins (e.g., VSV-G) may cause mild immune responses | Disadvantages: Pre-existing capsid antibodies (e.g., AAV2) in some populations may affect infection efficiency | |
| Loading Capacity | Advantages: Large loading capacity (approximately 7-8kb); can accommodate multiple shRNAs or long dsRNAs, suitable for complex RNAi systems | Advantages: Moderate loading capacity (approximately 4-5kb), meeting most RNAi needs (single shRNA/miRNA) | Advantages: None |
| Disadvantages: Exceeding capacity reduces packaging efficiency | Disadvantages: Overly long foreign genes affect viral titer and integration efficiency | Disadvantages: Very small loading capacity (only approximately 4.7kb); cannot accommodate large RNAi elements, requiring simplified vector design | |
| Expression Durability | Advantages: None | Advantages: Integrates into the host genome; stably transmitted with cell division; long-term expression (several months to years in vitro, long-term stability in vivo), suitable for long-term RNAi | Advantages: Stably exists as episomes in non-dividing cells (neurons/cardiomyocytes); long-term expression (months to years in vivo), suitable for long-acting RNAi |
| Disadvantages: Does not integrate into the host genome; exists as episomes; lost with cell division; short expression duration (1-2 weeks in vitro, 2-4 weeks in vivo), suitable for short-term RNAi | Disadvantages: No obvious disadvantages | Disadvantages: Diluted in dividing cells; short expression duration (about several weeks) | |
| Genome Integration | Advantages: Does not integrate into the host genome; no risk of insertional mutation; high safety for short-term applications | Advantages: Integration ensures long-term expression | Advantages: Almost no integration (integration frequency 10⁻⁵-10⁻⁶); mainly exists as episomes; insertion mutation risk is negligible; highest safety |
| Disadvantages: None (non-integration is the reason for short-term expression, not a defect) | Disadvantages: Randomly integrates into the host genome; may insert near proto-oncogenes or tumor suppressor genes, with potential carcinogenic risk (low probability) | Disadvantages: None | |
| Suitable Scenarios | hort-term, efficient RNAi experiments (e.g., transient knockdown in vitro cells, acute disease models) | Long-term, stable RNAi (e.g., establishing gene-knockdown cell lines, transgenic animal models, RNAi in hematopoietic stem cells) | in vivo long-acting RNAi (e.g., RNAi therapy for neurological and muscle tissue diseases), especially scenarios emphasizing safety and low immunogenicity |
With years of experience in gene therapy research and a focus on the study of human diseases, Creative Biolabs maintains a professional adenoviral vector construction platform. This platform is designed to support the development of gene therapy strategies, providing valuable assistance for researchers working in the realm of RNAi delivery.
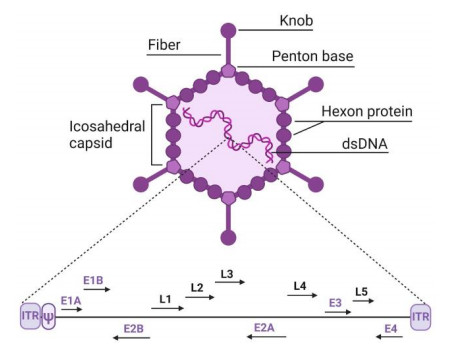 Figure 1. Structure of adenovirus and its genome1,2
Figure 1. Structure of adenovirus and its genome1,2
Tailored RNAi Vector Solutions
Customized Vector Component Design
Based on the customer's RNAi delivery needs, we optimize key adenoviral vector components to ensure efficient expression and targeted delivery of RNAi molecules:
Promoter Selection: We offer tissue-specific promoters (such as the liver albumin promoter and the muscle CK8 promoter), inducible promoters, and broad-spectrum promoters (such as CMV and EF1α) to meet the needs of targeted expression in different cells/tissues.
RNAi Molecule Insertion Optimization: We design optimized stem-loop structures (such as the miR-30a backbone), terminators (SV40 polyA, bGH polyA), and insulator elements for the siRNA/shRNA sequence to minimize off-target effects and enhance expression stability.
Reporter Gene Fusion: We can incorporate reporter genes such as GFP, RFP, or Luciferase, facilitating real-time monitoring of vector transduction efficiency and RNAi silencing efficacy through fluorescence imaging or chemiluminescence.
Safety Element Integration: We can insert toxic gene suppressor sequences and replication-defective E1/E3 The region deletion design significantly reduces the immunogenicity and cytotoxicity of the viral vector.
Virus Packaging and Purification
A third-generation adenovirus packaging system (deficient in the E1, E2A, and E4 regions) combined with an optimized 293A cell transfection system ensures high-titer and high-purity virus production:
Packaging Process: This covers the entire process, including plasmid construction and identification, cell plating, lipofectamine/viral transfection, viral amplification, freeze-thaw cycle harvesting, and ultracentrifugation purification.
Titer Control: The final viral titer can reach 10^10-10^12 PFU/mL (plaque-forming units), meeting the requirements of in vitro cell experiments (10^6-10^8 PFU/well) and in vivo animal experiments (10^9-10^11 PFU/animal).
Purity Testing: SDS-PAGE electrophoresis and HPLC analysis ensure viral particle purity >95% and endotoxin content <0.1 EU/μg to avoid nonspecific damage to cells or animals.
Quality Control and Validation Services
To ensure vector safety and RNAi efficacy, we provide multi-dimensional quality testing and functional validation:
Molecular Validation: PCR is used to verify vector genome integrity, and sequencing is used to confirm the correct insertion of the RNAi molecule.
Functional Validation: Complementary cell transduction experiments (e.g., HeLa, 293T, and primary hepatocytes) are provided. Target gene mRNA silencing efficiency is measured by qPCR (typically 50%-90%), and protein expression inhibition is measured by Western blot.
Safety Testing: Viral vector replication capacity is tested (plaque assay to verify the absence of replicating virus contamination), cytotoxicity (CCK-8 assay to determine post-transfection cell viability >80%), and immunogenicity (ELISA to measure expression levels of inflammatory factors such as IL-6 and TNF-α).
Personalized Technical Support
Solution Consultation: Experienced molecular biologists provide one-on-one technical consultation, recommending the optimal vector design based on the client's research objectives (e.g., tumor-targeted RNAi therapy, gene silencing for neurodegenerative diseases).
Data Analysis: Assist clients in interpreting experimental data from qPCR, Western Blot, animal imaging, and other studies, providing optimization recommendations.
Follow-up Services: We can extend our support to include the construction of stable RNAi cell lines and in vivo transduction of animal models (mice, rats, and non-human primates), forming a closed-loop support system of "vector design - preparation - validation - application."
Why Choose Our RNAi Vector Services?
Efficiency: Accelerate RNAi Research
Shorten R&D Cycles: The standard service process (vector design - packaging - purification - quality control) takes only 2-3 weeks, 50% shorter than the industry average (4-6 weeks).
High Silencing Efficiency: Through promoter optimization and RNAi sequence design, target gene silencing efficiencies can reach an average of over 70%, with some targets achieving over 90% inhibition, meeting the needs of demanding research.
Safety: Reduce Experimental Risk
Replication-Deficient Design: Utilizes a third-generation adenoviral vector with multiple deletions of the E1/E3/E4 regions, lacking the ability to replicate autonomously, thus preventing experimental contamination caused by viral spread.
Low Immunogenicity: Capsid protein modifications (such as PEGylation and RGD peptide modification) reduce the ability of the adenovirus to activate the host immune system, minimizing inflammatory responses during in vivo use, making it particularly suitable for long-term RNAi research.
Customization: Meeting Diverse Needs
Flexible Adaptation to Research Scenarios: Different vector types (e.g., cilia-modified, targeting ligand-conjugated) can be designed for in vitro cell-based experiments (adherent/suspension cells), in vivo local injection (intratumoral, muscle, ocular), or systemic administration (intravenous).
Multiple Format Options: Viral products are available in various volumes (100 μL, 1 mL, 10 mL) and titers, supporting a wide range of needs, from small-scale pilot studies to large-scale preclinical studies.
Expertise: Backed by a Proven Technology Platform
Experienced Team: Core technical personnel possess over 10 years of experience in viral vector R&D and have completed over 500 adenoviral vector design projects across a variety of fields, including oncology, cardiovascular medicine, and neuroscience.
GMP-Grade Production System: Viral packaging and purification are performed in a Class 10,000 cleanroom, complying with Good Manufacturing Practice (GMP) requirements, enabling the provision of compliant products for preclinical research.
Service Application Areas
Basic Research
Gene Function Research: By specifically silencing target genes, we analyze their roles in physiological processes such as cell proliferation, differentiation, and apoptosis (e.g., silencing the p53 gene to study its effect on tumor cell apoptosis);
Disease Mechanism Analysis: By silencing pathogenic genes (e.g., APP, tau protein genes) in cell models (e.g., neuronal cell models, hepatocyte models) or animal models (e.g., diabetic mice, Alzheimer's rats), we investigate the mechanisms of disease development.
Drug Discovery
Drug Target Validation: By silencing candidate drug targets in animal models, we observe changes in disease phenotypes (e.g., silencing the VEGF gene to verify its effect on tumor angiogenesis), and assess the druggability of the target.
RNAi Drug Development: We provide preclinical vector design and preparation services for RNAi drugs (e.g., shRNA drugs), helping them advance to the IND filing stage (e.g., developing RNAi drug vectors targeting the hepatitis B virus HBV gene).
Preclinical Research Areas
Cancer Therapy Research: Designing adenoviral vectors that target tumor cells (e.g., using tumor-specific promoters to drive shRNA expression) to silence oncogenes (e.g., MYC, KRAS), enhancing anti-tumor efficacy in combination with chemotherapy or immunotherapy.
Genetic Disease Research: Exploring gene therapy strategies by delivering siRNA via adenoviral vectors to silence the abnormal expression of mutant genes in animal models of rare diseases (e.g., cystic fibrosis mice).
References
- Muravyeva, Anna, and Svetlana Smirnikhina. "Adenoviral vectors for gene therapy of hereditary diseases." Biology 13.12 (2024): 1052. https://doi.org/10.3390/biology13121052
- Distributed under Open Access license CC BY 4.0, without modification.
