All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
CAR IVT mRNA in Cancer Immunotherapy
Viral delivery vector-mediated transfection could cause insertional mutagenesis that limits their applications in cancer immunotherapy. Due to the safety and facile production, in vitro-transcribed (IVT)-mRNA is an alternative for transient expression of chimeric antigen receptors (CARs) in human T cells and NK cells. Based on our proprietary production platform, Creative Biolabs is committed to offering high-quality CAR IVT products covering plasmid, mRNA, and LNP products.
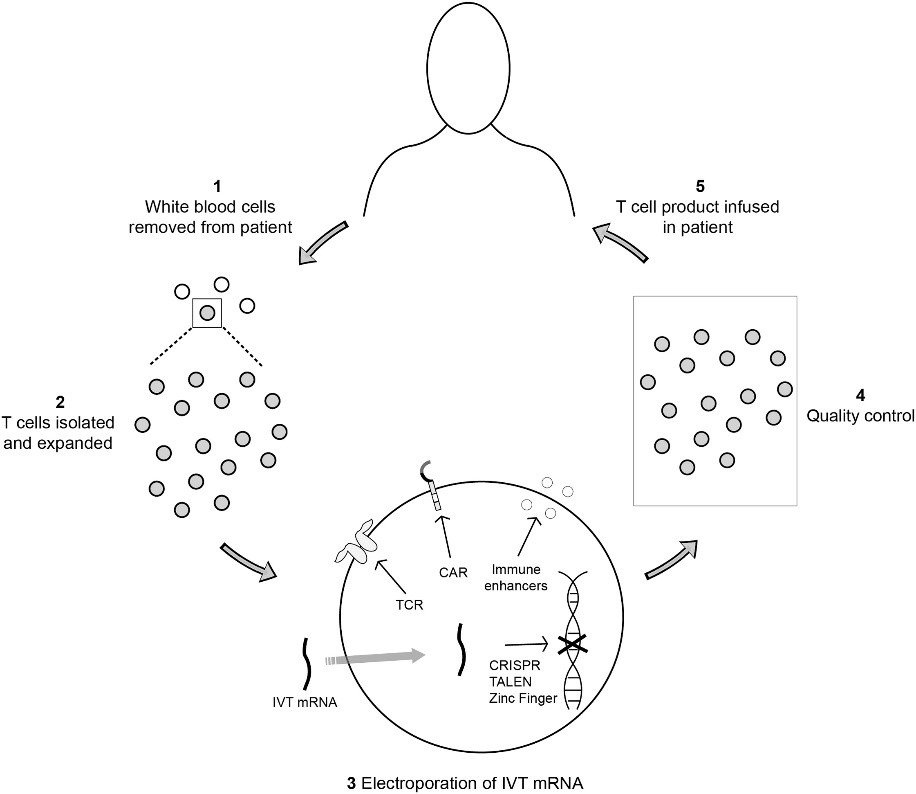 Fig.1 IVT mRNA application.1
Fig.1 IVT mRNA application.1
CAR IVT mRNA Products
Creative Biolabs is committed to improving our products and services to meet the growing requirements. Our team is experienced in IVT process development and help accelerate your project significantly. We provide a full range of CAR IVT mRNA products for the development of CAR-based therapeutics. Our mRNA products go through a series of rigorous quality control assays to ensure high quality.
CAR IVT mRNA Service
Creative Biolabs offers CAR IVT mRNA products and services to suit your needs. CAR IVT mRNAs can be custom-made to meet your specific requirements. A dedicated team of experts with years of experience will be assigned to your project for guidance and support. Our IVT mRNA services are flexible and can be customized according to your requirements. You can provide the cDNA your lab has at hand or the sequence of the gene of interest. Our team will be able to help you with mRNA manufacturing through the process of transcription so that you can focus on the discovery and innovation.
Our experts help design your IVT mRNA if desired. The project can start from various sources:
Please contact us to discuss your specific CAR IVT mRNA manufacturing needs.
Workflow
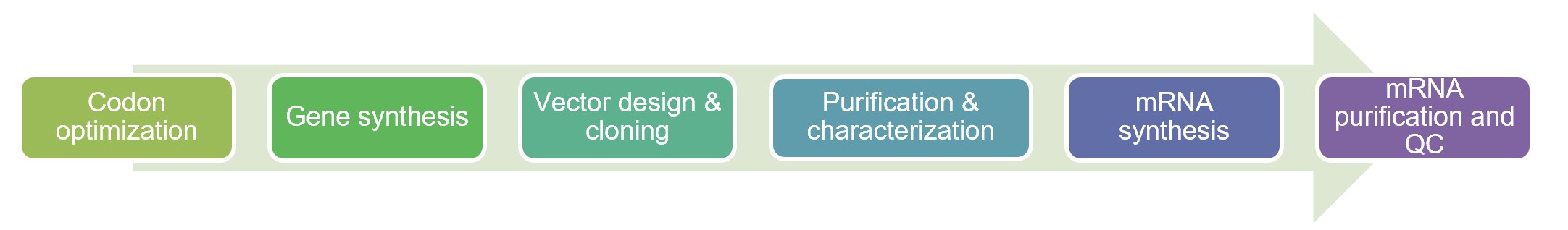 Fig.2 Workflow of IVT mRNA manufacture. (Creative Biolabs)
Fig.2 Workflow of IVT mRNA manufacture. (Creative Biolabs)
Reference
Associated Antigen Target
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION