Bioconjugation for Glycoconjugate Vaccines
Introduction of Glycoconjugate Vaccines
Vaccination is recognized as one of the most cost-effective and efficient methods for preventing and controlling infectious diseases, playing a crucial role in safeguarding public health. By significantly reducing the risk of disease transmission within communities, vaccines provide indirect protection to unimmunized populations when vaccination coverage reaches a sufficiently high level, achieving the concept of "herd immunity". According to the World Health Organization (WHO), over 20 high-risk diseases can be effectively prevented through vaccination, including influenza, human papillomavirus (HPV), herpes zoster, varicella, and rotavirus infections.
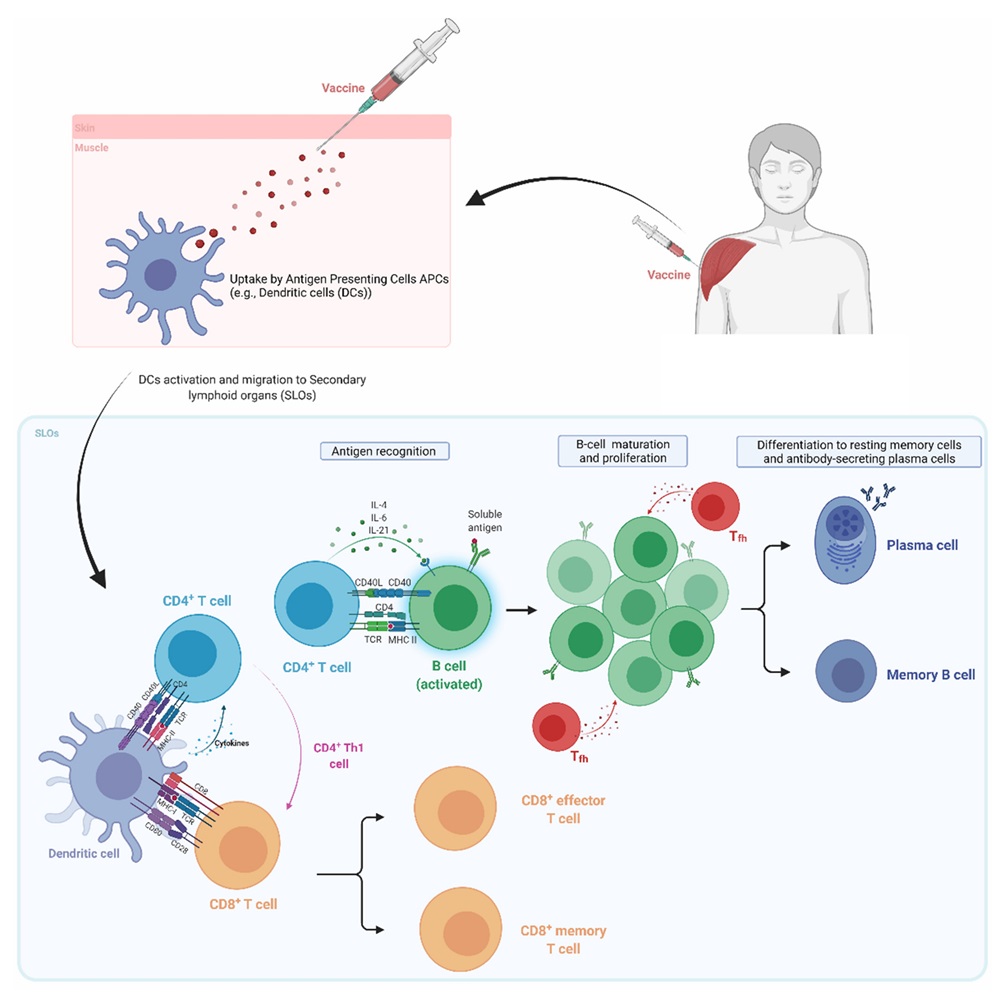 Fig.1 Mechanisms of vaccine-induced immune responses.1,4
Fig.1 Mechanisms of vaccine-induced immune responses.1,4
Over several centuries, vaccine platforms have undergone continuous innovation, now advancing to the use of Live attenuated vaccines and glycoconjugate vaccines. The cell surfaces of pathogens are densely coated with oligosaccharides and polysaccharides, which are vital virulence factors as they mediate receptor binding to host cells for initial adhesion and biological invasion. This makes them attractive targets for vaccine design. However, polysaccharide-based vaccines exhibit poor immunogenicity in immunocompromised individuals, leading to limited clinical efficacy. In contrast, glycoconjugate vaccines enhance the immunogenicity of polysaccharides by linking them to carrier proteins, proving particularly effective for children under two years old. These vaccines stimulate the immune system to produce polysaccharide-specific IgG antibodies and memory B cells, providing sustained immune protection.
Conjugation Approaches and Design of Glycoconjugate Vaccines
The conjugation between polysaccharides and proteins can be achieved through the functional groups of carbohydrates, such as carboxyl or hydroxyl groups, or by employing derivatized polysaccharides that facilitate the introduction of specific groups, like thiols or bromo groups. This approach effectively minimizes steric hindrance between the protein and the carbohydrate, enhancing the efficiency of the coupling process.
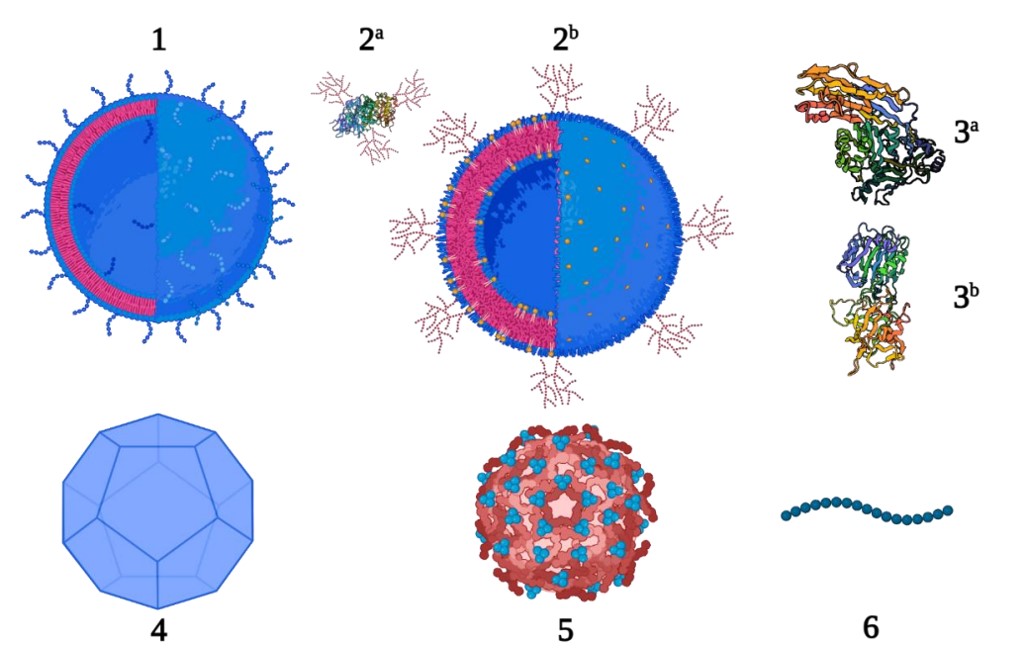 Fig.2 New carrier proteins for conjugate vaccines.2,4
Fig.2 New carrier proteins for conjugate vaccines.2,4
 Sodium periodate (NaIO4) oxidation
Sodium periodate (NaIO4) oxidation
NaIO4 oxidation is one of the most common strategies for the formation of aldehydes to facilitate conjugation. Aldehydes can directly engage in reductive amination reactions with lysine residues in proteins (containing ε-amino groups), or they can be further derivatized into other functional groups for protein conjugation. Additionally, oligosaccharides can directly conjugate with proteins through terminal reducing sugars.
 Activation of polysaccharides by cyanide
Activation of polysaccharides by cyanide
1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) is an organic cyano reagent utilized for the activation of hydroxyl groups in various polysaccharides, thereby enhancing subsequent condensation reactions with amines in carrier proteins.
 Conjugation based on unnatural amino acids (UAAs)
Conjugation based on unnatural amino acids (UAAs)
Engineering protein through the incorporation of UAA presents a powerful approach for generating carrier proteins with specific groups for efficient conjugation. For instance, the inverse demand Diels-Alder reaction (IEDDA) between tetrazine-functionalized glycans and target proteins (genetically encoded with trans-cyclooctene-modified UAAs) enables site-specific and biocompatible protein glycosylation in cell culture media or lysates.3
 Vaccine design based on nanoparticles
Vaccine design based on nanoparticles
In the development of novel glycoconjugate vaccines, liposomes and gold nanoparticles (AuNPs) are increasingly utilized as alternatives to conventional protein carriers. Liposomes are favored for their biocompatibility and low inherent immunogenicity, serving as effective co-delivery vehicles for polysaccharides and proteins. This can be accomplished by encapsulating polysaccharides within the liposome while modifying the surface with carrier proteins, or by modifying the surface of the liposome with chemically assembled glycopeptides. Additionally, AuNPs can be easily functionalized through Au-S bonds, facilitating the synthesis of fully synthetic carbohydrate vaccines.
Creative Biolabs possesses advanced technological platforms and extensive production experience, providing comprehensive solutions for the early development of glycoconjugate vaccines. We remain closely attuned to industry trends, continually innovating and enhancing our related technologies to better serve our clients and facilitate the efficient advancement of glycoconjugate vaccine research and development. Please contact us for more details.
References
- Ghattas, Majed, et al. "Vaccine technologies and platforms for infectious diseases: Current progress, challenges, and opportunities." Vaccines 9.12 (2021): 1490.
- van der Put, Robert MF, Bernard Metz, and Roland J. Pieters. "Carriers and antigens: new developments in glycoconjugate vaccines." Vaccines 11.2 (2023): 219.
- Machida, Takuya, et al. "Site-specific glycoconjugation of protein via bioorthogonal tetrazine cycloaddition with a genetically encoded trans-cyclooctene or bicyclononyne." Bioconjugate chemistry 26.5 (2015): 802-806.
- Distributed under Open Access license CC BY 4.0, without modification.
