Custom Polymer-Ligand Conjugation Service
Polymer-ligand conjugation technology is revolutionising biopharmaceutical development by precisely modulating the pharmacokinetics and targeting of biomolecules. Creative Biolabs offers comprehensive end-to-end customized polymer-ligand conjugation services with its team of experts possessing interdisciplinary expertise in synthetic chemistry, biochemistry, and analytical science.
Polymer Conjugation Introduction
In the biomedical field, combining the specificity of therapeutic or diagnostic molecules with their ideal behavior in vivo has always been a core challenge. Many promising candidates, such as therapeutic proteins, peptides, and nucleic acids, often fail due to rapid renal clearance, enzymatic degradation, immunogenicity, or nonspecific distribution. Polymer-ligand coupling technology has emerged to address this challenge. It precisely links ligands with specific biological functions to synthetic or natural polymers via covalent bonds, constructing novel bioconjugates and thus building a molecular bridge connecting in vitro and in vivo efficacy.
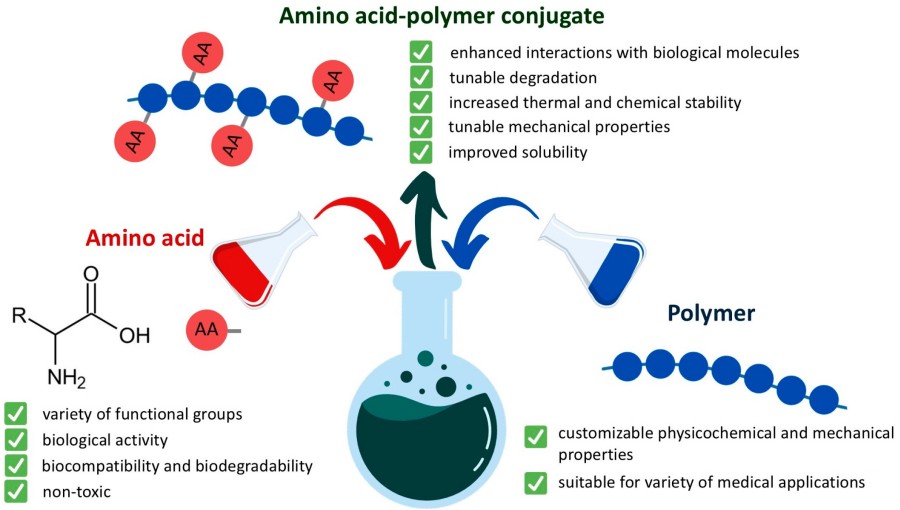 Figure 1. A general overview of the preparation of polymer-drug conjugate-based theranostic nanoparticles.1
Figure 1. A general overview of the preparation of polymer-drug conjugate-based theranostic nanoparticles.1
Structure of Polymer-Ligand Conjugates
Polymer-ligand conjugates are chimeric structures composed of the following core components linked by covalent bonds:
- Polymer Backbone: Serving as a carrier, it determines the physicochemical properties of the conjugate (e.g., water solubility, hydrodynamic radius). Common polymers include polyethylene glycol (PEG), N-(2-hydroxypropyl) methacrylamide (HPMA), peptides, or dextran.
- Ligand: The component with biological activity or diagnostic function, such as small molecule cytotoxic drugs (e.g., doxorubicin), nucleic acids (e.g., siRNA), proteins, or imaging probes.
- Linker: The chemical bridge connecting the polymer and the ligand; its design determines the drug release rate and targeting specificity.
Find the Right Services for Your Research
Ligand-polymer conjugates Our small molecule ligand-polymer conjugates can be used for drug delivery and receptor-specific imaging, thereby achieving selective targeting.
Custom Polymer Synthesis and Functionalization
We synthesize and functionalize a variety of polymers (PEG, pHPMA, PGA, dextran) with reactive end groups (e.g., maleimide, NHS ester, DBCO, azides, vinyl sulfone, thiols) for site-specific conjugation.
Ligand Engineering and Modification
We can engineer and modify your targeting ligands (e.g., antibody fragmentation, peptide synthesis, introduction of non-natural amino acids) to introduce specific functional groups for conjugation.
Standard Polymer-Ligand Conjugation
We employ innovative polymer-ligand coupling methods to provide comprehensive solutions for researchers and developers seeking to enhance the performance and efficacy of biomolecules.
Multi-Ligand and Multi-Arm Polymer Conjugates
Construct complex systems that link multiple different ligands on a single polymer (for multi-target therapy) or multiple arms (e.g., 4-arm, 8-arm PEG) or branched polymer scaffolds to achieve high affinity.
Polymer-Ligand-Drug Conjugates
Integrate the synthesis of complete therapeutic conjugates where the drug payload is attached to the polymer backbone along with the targeting ligand, forming a complete "magic bullet" structure.
Advanced Conjugation Strategies
We employ a series of advanced bioconjugation chemistry techniques to ensure the stability and purity of the resulting conjugates:
Click Chemistry
Click chemistry has become the gold standard for constructing complex conjugates due to its high efficiency, high specificity, and bioorthogonality.
- Cu(I)-catalyzed azido-alkyne cycloaddition (CuAAC): A classic "copper-catalyzed" reaction involving the introduction of an azide group onto the polymer backbone and an alkyne group onto the ligand. The copper catalyst enables rapid formation of a stable triazole ring under mild conditions.
- Strain-promoted azido-alkyne cycloaddition (SPAAC): Utilizing highly strained cycloalkynes (e.g., DBCO), this method eliminates the need for toxic copper catalysts. It is suitable for the conjugation of cytotoxic biomolecules such as proteins.
Thiol-maleimide Conjugation
This is one of the most commonly used and reliable methods in bioconjugation. The thiol (-SH) group (typically derived from the cysteine side chain) reacts rapidly with the maleimide group under mild pH conditions to form a stable thioether bond. We precisely control the reaction conditions to minimize side reactions such as maleimide hydrolysis and thiol exchange.
Enzyme-Cleavable Linkers
To achieve precise intratumoral drug release, we provide a variety of enzyme-cleavable linkers, such as peptide linkers. For instance, the classical Gly-Phe-Leu-Gly (GFLG) sequence is specifically recognized and hydrolyzed by lysosomal enzymes such as Cathepsin B. This design ensures that the conjugate remains stable in plasma but enables rapid release of the active drug within enzyme-rich tumor cells following endocytosis.
Creative Biolabs Polymer Selection Guide
Choosing the right polymer is crucial for the successful construction of polymer-ligand conjugates. The physicochemical properties of the polymer directly determine the pharmacokinetics, biodistribution, degradation behavior, and ultimate biological function of the conjugate. To meet your diverse R&D needs, we offer a range of well-validated polymer platforms. The table below details the key characteristics of our core polymer library to guide you in making the best choice.
| Polymer | Key Characteristics | Major Advantages | Ideal Application Scenarios |
|---|---|---|---|
| Polyethylene Glycol (PEG) | "Stealth" polymer, hydrophilic, flexible, uncharged. |
|
|
| Poly(N-(2-hydroxypropyl)methacrylamide) (pHPMA) | Biodegradable, non-immunogenic, highly functionalizable synthetic polymer. |
|
|
| Poly(L-glutamic acid) (PGA) | Biodegradable, anionic, natural-derived polymer with high loading capacity. |
|
|
| Dextran | Natural, biodegradable, hydrophilic polysaccharide. |
|
|
| Poly(L-lysine) (PLL) | Cationic, backbone-degradable poly(amino acid). |
|
|
| Polyethylenimine (PEI) | High cationic charge density "proton sponge" polymer. |
|
|
| Poly(N-isopropylacrylamide) (PNIPAM) | "Smart" thermosensitive polymer with a Lower Critical Solution Temperature (LCST). |
|
|
Our Expert Guidance
The table above is for reference only. Our team of scientists will provide more in-depth consultation based on your specific goals (e.g., in vivo targeting efficiency, drug release kinetics, regulatory pathways for the end application) to recommend the most suitable polymer or custom copolymer, thereby maximizing the success rate of your project.
Our Collaboration Process
Creative Biolabs employs a systematic and transparent four-stage process to ensure the successful and efficient completion of every custom polymer-ligand conjugation project.
![]()
Phase I: Consultation and Design
Our PhD-level scientists consult with clients to determine the Target Product Profile (TPP). This includes determining the polymer type, molecular weight, linker chemistry, desired drug-to-polymer ratio (DPR), and required purity/yield. We utilize computational models to predict optimal stoichiometry and reaction conditions.
![]()
Phase II: Synthesis and Conjugation
Based on the TPP, the polymer is functionalized, and the conjugation reaction is performed. This involves iterative optimization of the solvent system, temperature, pH, and reaction time to maximize conjugation yield while minimizing byproduct formation.
![]()
Phase III: Purification and Separation
The crude product is purified using advanced purification techniques, typically size exclusion chromatography (SEC), ion exchange chromatography (IEX), or tangential flow filtration (TFF), to separate pure monodisperse conjugates.
![]()
Phase IV: Quality Control and Documentation
The final product undergoes rigorous quality control (QC) testing (see Section 6) and a comprehensive report containing all analytical data is provided to the customer.
Quality Control of Polymer-Ligand Conjugates: Ensuring Efficacy and Safety
We uphold the philosophy that "quality stems from design." Our quality control process encompasses multiple aspects:
- ✅Purity Analysis: High-performance liquid chromatography (HPLC) and size exclusion chromatography (SEC) are used to separate and quantify conjugates from unreacted starting materials and aggregates.
- ✅Structural Validation: Mass spectrometry analysis (MALDI-TOF, LC-ESI-MS) confirms molecular weight and provides evidence of successful conjugation.
- ✅Functional Integrity Assay: We verify whether the conjugated ligand retains its biological function. This may include: Surface plasmon resonance (SPR) or biolayer interference (BLI) measurements of binding kinetics with the target receptor (KD, Kon, Koff).
Frequently Asked Questions
Q: How can I determine if my ligand remains active after conjugation?
A: We strongly recommend that you provide a range of functional assays (SPR, BLI, cell assays) as part of our characterization protocol. This is the ultimate method to verify the success of the conjugation.
Q: My target is intracellular. Can your conjugates facilitate delivery?
A: Yes. By selecting ligands capable of mediating receptor endocytosis (e.g., folic acid, transferrin) and integrating lysosomes or cleavable linkers into the polymer design, we can construct highly efficient intracellular delivery conjugates.
Q: Can you conjugate my ligand to a specific site on a protein?
A: Yes, we specialize in site-specific coupling, using methods such as aldehyde tag modification, enzymatic ligation, or conjugation to N or C terminal residues to ensure protein activity is preserved.
Conclusion
The future of biomedicine lies in precise targeting and controlled delivery, and custom polymer-ligand conjugation is fundamental to achieving these goals. Creative Biolabs possesses the necessary scientific expertise, advanced technology, and rigorous quality control to transform your novel ligands into viable therapeutic or diagnostic drug candidates. Partner with us to leverage the power of polymer chemistry and accelerate your biomedical discoveries. Please contact us to discuss your demands or to request a proposal.
Reference
- Manandhar S, Sjöholm E, Bobacka J, et al. Polymer-drug conjugates as nanotheranostic agents. Journal of Nanotheranostics, 2021, 2(1): 63-81. https://doi.org/10.3390/jnt2010005 Distributed under Open Access license CC BY 4.0, without modification.
