Custom Oligonucleotide-Nanoparticle Conjugation Service
Nanoparticle-Oligonucleotide Conjugation Introduction
Nucleic acid-polymer conjugates are the convergent point of nanotechnology and molecular biology. The covalent or non-covalent conjugation of oligonucleotide payloads to nanoparticle scaffolds results in single, multifunctional nanostructures. This approach has a double benefit: the nanoparticle allows for a stable and protected oligonucleotide carrier and the conjugated oligonucleotides can be used as targeting moieties or as therapeutic agents. This approach also helps to overcome limitations of free nucleic acid delivery such as the rapid degradation by nucleases and the inability to cross biofilms because of their anionic charge. These complexes have a higher stability, improved pharmacokinetics and a higher ability for targeted delivery to specific cell types or tissues in a physiological environment.
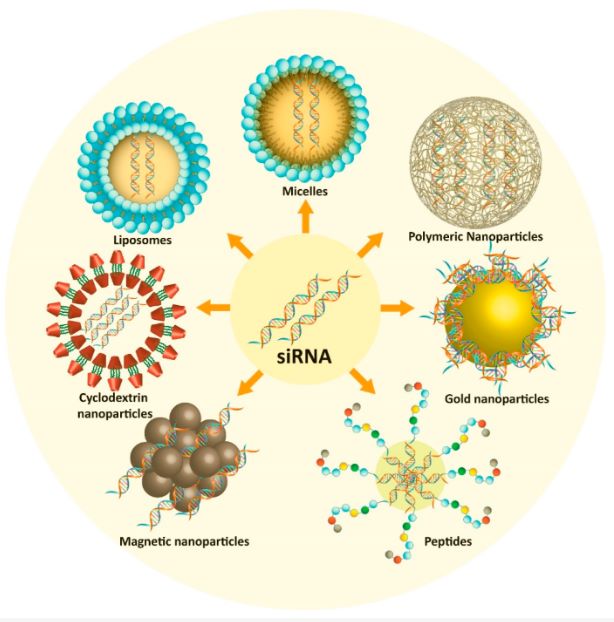 Figure 1 Nanostrategies used for siRNA delivery in effective prostate cancer therapy.1
Figure 1 Nanostrategies used for siRNA delivery in effective prostate cancer therapy.1
Nanoparticle-siRNA Conjugation
Small interfering RNA (siRNA) is the cornerstone of RNA interference (RNAi), a natural biological process that silences gene expression in a sequence specific manner. In terms of treatment, siRNA can be used to "shut down" pathogenic genes, making it a promising therapeutic approach for various diseases ranging from cancer to viral infections. Nanoparticle coupling provides an ideal solution that protects siRNA payloads from damage by endonucleases and promotes their uptake by cells through endocytosis. There are various conjugation strategies, including:
Covalent coupling: involves the formation of stable chemical bonds between the surface of nanoparticles and siRNA. Common methods include click chemistry, maleimide thiol coupling, or carbodiimide chemistry.
Non covalent binding: relies on electrostatic interactions between negatively charged siRNA and positively charged nanoparticles (such as polymer complexes or lipid complexes).
siRNA-Loaded Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) are submicron sized colloidal carriers composed of a stable solid lipid matrix at both room temperature and body temperature. Due to their biocompatibility, biodegradability, and the ability to be manufactured using mature technologies, they are highly attractive in siRNA delivery. For siRNA encapsulation, SLN usually carries a positive surface charge to attract negatively charged siRNA through electrostatic forces. SiRNA can be encapsulated within a lipid matrix or adsorbed onto the surface of nanoparticles.
Nanoparticle mRNA Conjugation
mRNA has been intensively studied as a potential therapeutic agent for vaccination and protein replacement therapy in recent years. It is more "active" than DNA in that it can directly operate in the cytoplasm without entering the nucleus. However, the large size and instability of mRNA make delivery in vivo a major challenge. Nanoparticle coupling provides a means to encapsulate and protect mRNA from ribonucleases, thus allowing it to be safely and efficiently delivered to the cytoplasm for endosome escape.
Nanoparticle mRNA Conjugation Design
The nanoparticles used for delivering mRNA are carefully designed to perform the following key functions
- Encapsulation: Nanoparticles need to encapsulate the mRNA completely to avoid its degradation.
- Targeting: Ligands can be added to the surface of nanoparticles to target specific types of cells, thus, delivering mRNA to the places where it is needed the most.
- Endosomal Escape: Cationic or ionizable lipids are generally used to mediate endosomal escape. In the acidic environment of the endosome, the protonation of cationic or ionizable lipids triggers a lipid conformational change, which disrupts the endosomal membrane.
Lipid Nanoparticle (LNP) mRNA Vaccine
The LNP used in mRNA vaccines is a highly formulated complex that is composed of four key lipid components.
- Ionizable Cationic Lipid
- Phospholipid
- Helper Lipid
- PEGylated Lipid
Overview of What Creative Biolabs Can Provide
Creative Biolabs offers custom synthesis of oligonucleotides and their coupling to nanoparticles. This is not a one size fits all service, we work with our clients to design, develop, and optimize tailored coupling strategies that are best suited for their research and treatment objectives. Our team of experts guarantee the highest quality and repeatability from initial consultation through to final product delivery. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Types of Nanoparticles
Our expertise covers multiple types of nanoparticles suitable for oligonucleotide coupling, each with unique advantages in specific applications:
 Gold Nanoparticle (AuNP)
Gold Nanoparticle (AuNP)
It is known for their unique optical properties, high surface area, and biocompatibility. They are commonly used for diagnosis and are easy to functionalize.
 Silver Nanoparticle (AgNP)
Silver Nanoparticle (AgNP)
It possesses antibacterial properties and can be used for biosensing and drug delivery.
 Polymer Nanoparticle
Polymer Nanoparticle
They are biodegradable and biocompatible, with a wide range of applications, and can be formulated to achieve controlled release of therapeutic payloads. For example, polylactic acid glycolic acid copolymer (PLGA) and polycaprolactone (PCL).
 Lipid Nanoparticles (LNP)
Lipid Nanoparticles (LNP)
Self-assembling vesicles composed of lipid bilayer membranes, which are highly suitable for encapsulating hydrophilic and hydrophobic molecules. They are the gold standard for nucleic acid delivery.
Discover Why Creative Biolabs Stands Out
- Skilled professionals with rigorous QA and QC processes
- State-of-the-art analytical equipment
- Expert technical assistance
- Personalized one-on-one customer support
- Competitive pricing
- Quick delivery
Frequently Asked Questions
Q: What factors determine the selection of nanoparticle platforms for specific applications?
A: The choice of nanoparticle platform depends on various factors, including the type of oligonucleotide (siRNA, mRNA, etc.), expected application (therapy, diagnosis, or treatment), administration route, target tissue or cell, required release kinetics, and manufacturing process.
Q: How to ensure the biological activity of oligonucleotide conjugation?
A: We adopt various strategies to maintain the activity of oligonucleotides, including optimizing coupling chemistry to avoid modification of key regions, using mild reaction conditions, and using protective groups when necessary. After conjugation, we will conduct rigorous functional assays to confirm biological activity, such as the gene silencing efficiency of siRNA conjugates or the protein expression level of mRNA conjugates.
Q: What is the typical cycle for customized coupling services?
A: The specific time depends on the complexity and specific requirements of the project. Simple coupling can be completed within 2-4 weeks, while more complex projects involving novel nanoparticle synthesis, extensive optimization, or biological testing may require 8-12 weeks or longer.
Q: Can you achieve targeted delivery to specific tissues or cells?
A: Yes, we offer various targeting strategies, including surface functionalization using antibodies, peptides, aptamers, or other targeting ligands to recognize specific biomarkers on target cells. We also utilize physiological targeting methods based on particle size and surface characteristics that affect biological distribution.
Reference
- Ashrafizadeh M, Hushmandi K, Rahmani Moghadam E, et al. Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer. Bioengineering, 2020, 7(3): 91.https://doi.org/10.3390/bioengineering7030091 Distributed under Open Access license CC BY 4.0, without modification.
