Custom CpG-siRNA Conjugation Service
What are CpG-siRNA Conjugates?
The development of RNA interference (RNAi)-based therapeutics offers an unprecedented ability to silence disease-associated target genes. To successfully apply RNAi in the clinic, two major hurdles have to be overcome: effective cell-specific targeting/delivery of siRNA and modulation of immune stimulation by nucleic acids. On the other hand, the immune cells can be important targets for cancer therapy themselves. In fact, the ligands of intracellular receptors have been found to be useful tools for cell-specific siRNA delivery. TLR9-positive cells recognize and endocytose single-stranded oligodeoxynucleotides (CpG ODNs) with unmethylated CpG motifs. CpG ODNs conjugated with Dicer substrate siRNA (CpG-siRNA) are recognized by human and mouse TLR9+ cells without any transfection reagent, and gene silencing of mouse TLR9+ immune cells (dendritic cells (DCs), macrophages, and B cells) in vitro and in vivo. It was shown in mouse clinical trials that CpG can directly or indirectly target several oncogenic factors such as antitumor active STAT3, STAT5, RELA/P65, BCL2L1, and S1PR1 via siRNA.
Table 1. Molecular Components of CpG-siRNA Conjugates
| Component | Composition | Biological Function | Mechanism of Action |
|---|---|---|---|
| CpG ODN | Unmethylated cytosine-guanine dinucleotides in specific sequence contexts | Immunostimulation | TLR9 receptor activation leading to NF-κB signaling and cytokine production |
| siRNA | 21-23 bp double-stranded RNA with 2-nt 3' overhangs | Gene silencing | Sequence-specific mRNA degradation via RNA-induced silencing complex (RISC) |
| Linker/Carrier | Chemical crosslinkers or nanoparticle platforms | Structural stability and delivery | Covalent conjugation or physical encapsulation ensuring co-delivery to target cells |
The Advantages of CpG-siRNA Conjugates
The therapeutic advantage of CpG-siRNA conjugates stems from their unique dual regulatory capacity, simultaneously modulating multiple disease pathways through complementary mechanisms. Unlike monotherapies that target single nodes in complex disease networks, these conjugates can simultaneously activate immune and gene regulatory mechanisms. The synergistic effect between the CpG and siRNA components can produce enhanced therapeutic effects that cannot be achieved by monotherapy. The immunostimulatory activity of the CpG motif can generate a local inflammatory microenvironment, thereby enhancing the sensitivity of target cells to gene silencing and recruiting immune effector cells, particularly in cancer treatment, where these cells contribute to disease control. The following are key advantages of CpG-siRNA conjugates:
✅Can overcome the shortcomings of small molecule drugs
✅Don't require any complex formulations or delivery vectors
✅Target both cancer and immune cells at the same time and thus increase the total effect
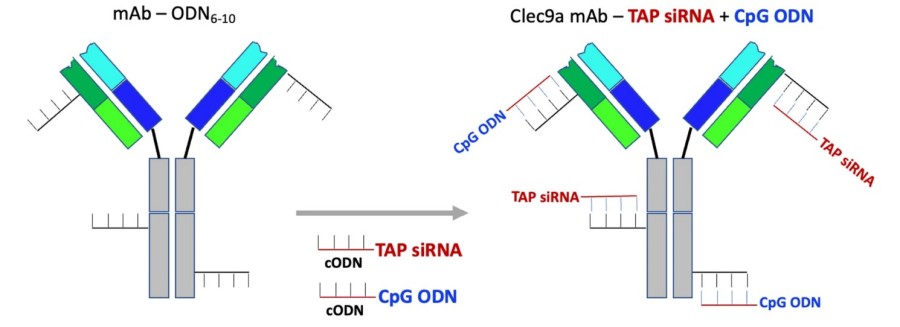 Figure 1 Conjugation of CpG ODN and TAP siRNA to the Clec9a antibody. An equimolar mixture of CpG ODN and TAP siRNA was hybridized to the oligo-modified Clec9a antibody.1
Figure 1 Conjugation of CpG ODN and TAP siRNA to the Clec9a antibody. An equimolar mixture of CpG ODN and TAP siRNA was hybridized to the oligo-modified Clec9a antibody.1
Technology of CpG-siRNA Conjugation
Successful synthesis of functional CpG-siRNA conjugates requires precise control over complex bioconjugation chemistry. The goal is to obtain molecules with high purity and well-defined structures.
Key Conjugation Strategies
Phosphoramide Chemistry (Solid-Phase Synthesis)
This is often the preferred method for oligonucleotide synthesis, allowing for the stepwise parallel synthesis of CpG ODN and siRNA chains. Linking molecules (e.g., non-nucleoside C3 or C6 spacers with functional groups such as amines or thiols) are directly inserted into the sequence, typically at the 5' or 3' end.
01. Thiol-Maleimide Coupling: This is the most commonly used and reliable method, utilizing an efficient Michael addition reaction between a thiol-modified oligonucleotide and a maleimide-modified ligand. This reaction forms a stable thioether bond.
02. Click Chemistry (Cu-catalyzed azide-alkyne cycloaddition reaction - CuAAC): A highly bioorthogonal and efficient method for linking azide-functionalized oligonucleotides with alkyne-functionalized ligands to form stable triazole rings.
What Creative Biolabs Offers?
Creative Biolabs provides an end-to-end CpG-siRNA conjugate service platform, including design, optimization, large-scale manufacturing and regulatory support. Our CpG-siRNA conjugate services start with conjugate design and optimization. Our scientists collaborate with clients to identify the best target sequence, CpG motif, conjugation strategy and delivery vehicle for the desired therapeutic use.
Customized siRNA Synthesis Services
- siRNA Sequence Selection
- Flexible siRNA Synthesis Scale
- siRNA Modification and Labeling
- Strict Quality Control
- Fluorescently labeled CpGs
- CpG-siRNA Conjugate Services
CpG ODN Services
- Natural CpGs that can be functionalized with linkers and bases or modifying groups
- Universal CpG solid-phase carriers for oligonucleotide synthesis
- Chemical modifications, such as the introduction of unmodified U, puromycin, unmodified inosine, base-free spacers, locked nucleic acid T analogs, and locked nucleic acid A analogs at the 3' end
CpG Quality Assay and Stability Analysis
- Construction and Purification of CpG-siRNA Conjugates
- Gene Silencing Effect Assessment Analysis
- Characteristics of CpG-siRNA Conjugates
Characterization of CpG-siRNA Conjugates
Creative Biolabs employs a comprehensive analytical framework to fully characterize the physicochemical and biological properties of CpG-siRNA conjugates, ensuring their quality, stability, and therapeutic potential. Our characterization process begins with physicochemical analysis to determine key quality attributes, including molecular size, composition, purity, and structural integrity.
| Analytical Parameter | Technique | Purpose |
|---|---|---|
| Molecular Mass & Purity | Mass Spectrometry (MS) (e.g., MALDI-TOF) & High-Performance Liquid Chromatography (HPLC) | Confirms the exact molecular weight and verifies the purity of the final conjugate, separating it from unreacted starting materials (free siRNA/CpG) and side products. |
| Structural Integrity | Polyacrylamide Gel Electrophoresis (PAGE) or Capillary Gel Electrophoresis (CGE) | Assesses the proper annealing of the siRNA duplex and the structural integrity of the final, larger conjugate molecule. |
| Biostability | Nuclease Digestion Assay (in serum) | Evaluates the resistance of the conjugate to enzymatic degradation in biological fluids, confirming enhanced in vivo stability. |
| Functional Activity (In Vitro) | Cellular Uptake Assays (e.g., Flow Cytometry with fluorescently labeled conjugates) & Gene Silencing Assay (e.g., qPCR, Western Blot) | Confirms targeted uptake by TLR9+ cells and measures the dose-dependent reduction of the target mRNA/protein. |
| Immunostimulatory Activity | Cytokine ELISA (in TLR9+ cell culture) | Quantifies the production of key pro-inflammatory cytokines (e.g., IFN-α, IL-6) to verify the functional activation of TLR9. |
Types of CpG-siRNA Conjugates
| Conjugate Type | Key Features | Advantages | Ideal Applications |
|---|---|---|---|
| Direct Covalent Conjugates | Simple structure, cleavable linkers, minimal modification | Rapid distribution, straightforward synthesis, reduced immune recognition | Systemic administration for immune cell targeting |
| Ligand-Targeted Conjugates | Tissue-specific ligands (e.g., GalNAc), spacer arms | Enhanced cellular uptake in target tissues, reduced off-target effects | Liver-specific diseases, precision medicine applications |
| Nanoparticle-Encapsulated Systems | Gold, polymeric, or lipid nanoparticles, PEGylation | High payload capacity, tunable release, combinatorial therapy | Oncology applications, complex tissue targeting |
Our Strengths
✅Scientific Expertise: Years of experience with the synthesis of complex, therapeutically relevant, oligonucleotide conjugates.
✅Customization & Flexibility: We offer both small-scale research batches, as well as large-scale, cGMP-compliant manufacturing for advanced preclinical programs.
✅Purity & Quality: We operate under the strictest quality control guidelines to provide our customers with the highest degree of reproducibility and the lowest off-target activity.
Frequently Asked Questions
Q: How long is the typical development cycle for CpG-siRNA conjugates?
A: For standard direct conjugates without additional chemistry or assembly steps, the typical development cycle from start to delivery can be anywhere from 4 to 8 weeks, while more complex, modular nanoparticle formulations can take 12 to 16 weeks, depending on multiple factors including project complexity, biomaterial and customization specifications, initial lead structure availability, and synthesis yield optimizations. The conjugate design phase, including 1-2 weeks of bioinformatics-based design and sequence optimization and 2-3 weeks of oligonucleotide synthesis and conjugation, is typically followed by 1-2 weeks of purification and full characterization, with optional 2-4 weeks of cell-based functional validation. Creative Biolabs has the capability to expedite the timeline for priority projects without sacrificing quality.
Q: How to ensure that gene silencing and immune activation are optimal?
A: We use an integrated optimization approach. For the siRNA design we use bioinformatics to screen siRNA oligos for a variety of factors, including GC content, size, thermodynamic asymmetry, PAM sequences, accessibility and specificity analysis, in order to design an siRNA with optimal silencing efficiency and minimal off-target effects. For the CpG part, we screen for effective TLR9 activating motifs in the species of interest. For the conjugation method, we determine the linking position and orientation of CpG relative to siRNA so that the functional groups of each component are not sterically blocked. Post-synthesis, its functionality is then validated with standardized in vitro biological assays, such as qRT-PCR for gene silencing and ELISA-based cytokine profiling for immune activation. Redesign is done iteratively to ensure the target performance is achieved.
Q: Can you attach specific targeting ligands?
A: Yes, at Creative Biolabs, we are experienced with designing and synthesizing CpG-siRNA conjugates and customizing these conjugates to include various homing ligands for tissue-specific delivery. We have particular experience in GalNAc conjugation to allow for the direct introduction of GalNAc during solid-phase synthesis with a customized CPG carrier to target hepatocytes. We can also use our conjugation platform to attach a variety of peptide ligands, antibody fragments, and other targeting moieties. We work closely with our clients to identify the most effective targeting ligand and strategies based on the target tissue accessibility, receptor expression profiles, and internalization efficiency, among other factors.
Q: What chemical modifications can be made to improve stability?
A: We provide a full range of modification options to increase nuclease resistance, enhance pharmacokinetics, and decrease immunostimulatory properties as needed. We commonly use 2'-O-methyl, 2'-fluoro, and 2'-O-methoxyethyl chemical modifications for siRNA oligos at specific positions to provide nuclease resistance without affecting RNAi activity. In addition to nucleotide-level modifications, we also provide options for backbone, or skeletal, modifications to CpG-siRNA conjugates, such as introducing phosphate thioester bonds to enhance protein binding in plasma and increase in vivo half-lives. For the CpG component, we use phosphate thioester stabilization as well as base modifications to modulate TLR9 activation potency. The types, numbers, and position targeting of chemical modifications are all optimized and based on extensive prior experience to provide the best therapeutic effects while minimizing potential side effects.
Q: Can you provide in vivo validation of the conjugates?
A: Creative Biolabs can provide in vivo validation services to determine the therapeutic efficacy, pharmacokinetics, and safety of CpG-siRNA conjugates in relevant disease models. The applications and disease models for our in vivo evaluation services include efficacy studies in subcutaneous, in situ, and metastatic tumor models for oncology, viral infection models, inflammatory and immune-mediated diseases, and genetic disease models. Our biodistribution studies with fluorescently or radiolabeled conjugates determine tissue targeting and penetration. We also offer standard and extended toxicological assessments in rodent and non-rodent species to support preclinical development. All in vivo studies are designed and performed according to regulatory guidelines to provide robust data for IND submissions.
Conslusion
The conjugation of CpG with siRNA (CpG-siRNA conjugates) is a novel therapeutic platform that aims to combine the desired on-target gene silencing effect with the potent immunomodulatory ability of CpG ODNs. Designing and synthesizing CpG-siRNA conjugates is a highly specialized process that requires a wide range of technical knowledge across various disciplines, including oligonucleotide chemistry, CpG bioactivity, immunology, siRNA therapeutics, and delivery. Creative Biolabs has been a pioneer in the field of CpG-siRNA conjugates and offers a comprehensive platform for conjugate development, including development strategies, technology platforms, and a wealth of experience. Don't hesitate to contact us!
Reference
- Clark E S, Benaduce A P, Khan W N, et al. Vaccination against neoantigens induced in cross-priming cDC1 in vivo. Cancer immunology, immunotherapy, 2024, 73(1): 9. https://doi.org/10.1007/s00262-023-03597-y Distributed under Open Access license CC BY 4.0, without modification.
