Custom DPC-siRNA Conjugation Service
What are Dynamic Polyconjugates?
The potential of siRNA to selectively silence specific genes has attracted widespread attention, making it suitable both as a research tool and as a treatment for a variety of diseases, including cancer, infectious diseases, and metabolic disorders. A key challenge in these siRNA-based applications is how to efficiently deliver siRNA to target cells in vivo. Therefore, researchers are developing various non-viral and viral vector systems for delivering siRNA to the liver, tumors, and other tissues. To minimize off-target delivery of siRNA to non-target cells in the liver, researchers modified the amino groups of amphiphilic polyvinyl ether (PBAVE) with maleic anhydride, creating acid-labile maleic acid bonds that dissolve in the acidic environment of the endosome. This process exposes the amino groups of the drug, thereby improving its endosome solubility. This delivery system, termed a siRNA dynamic polymer conjugate, is characterized by the reversible attachment of siRNA, a shielding agent, and a targeting ligand to a polymer, the polymer's endosome solubility of which is activated by its chemical environment.
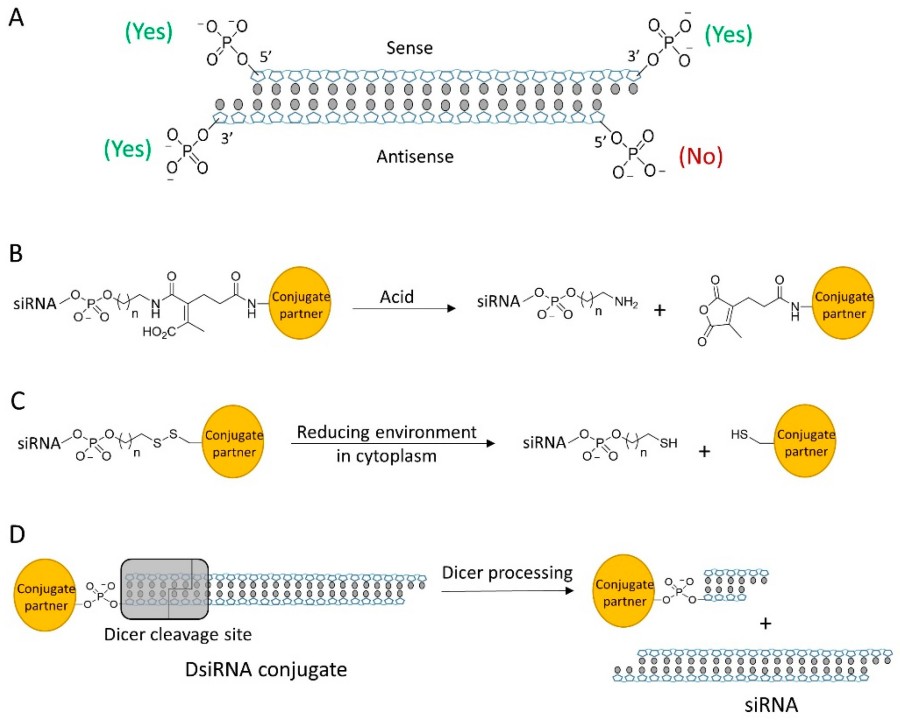 Figure 1 (A) siRNA contains 4 terminal phosphate groups for modification, but only 3 sites are tolerable for bioconjugation. (B) Acid-sensitive linker cleaved by acid. (C) Disulfide linker can be cleaved in cytosol to release siRNA. (D) DsiRNA conjugate is processed by Dicer to release mature siRNA.1
Figure 1 (A) siRNA contains 4 terminal phosphate groups for modification, but only 3 sites are tolerable for bioconjugation. (B) Acid-sensitive linker cleaved by acid. (C) Disulfide linker can be cleaved in cytosol to release siRNA. (D) DsiRNA conjugate is processed by Dicer to release mature siRNA.1
Structure of Dynamic Polyconjugates
DPC is a complex macromolecular assembly whose structure is based on a membrane-active polymer backbone that serves as the primary carrier. This backbone is reversibly linked to three key functional components.
01. Therapeutic carriers: Typically, oligonucleotides, such as siRNA, ultimately released into the reducing environment of the cytoplasm via reversible linkages (e.g., disulfide bonds).
02. Targeting ligands: A molecule, such as N-acetylgalactosamine (NAG) for hepatocyte targeting, that promotes active and cell-specific uptake via receptor-mediated endocytosis.
03. Shielding agents: Polyethylene glycol (PEG), reversibly linked via pH-sensitive linkages (e.g., maleamic acid bonds derived from carboxydimethylmaleic anhydride (CDM)).
DPC-siRNA Conjugation
The most classic and clinically validated form of the DPC platform is the delivery of siRNA (small interfering RNA). siRNA is an effective tool for gene silencing via RNA interference pathways, with potential for treating metabolic disorders, infectious diseases, and cancer.
siRNA Stabilization Technology
Highly efficient siRNA dynamic polymer conjugates enhance nuclease resistance and prolong activity through extensive chemical modifications. Advanced modification modalities such as alternative 2'-O-methylation (Alt OMe), enhanced stabilization chemistry (ESC), and advanced ESC (Adv ESC) have been shown to significantly improve serum stability.
siRNA-polymer Linkage
siRNA is typically linked to a PBAVE polymer via reduction-sensitive disulfide bonds. The concentration of reducing agents (e.g., glutathione) in the cytoplasm is much higher than in the extracellular space or endosomes, ensuring that siRNA is cleaved and released only after escaping the endosome.
Shielding/targeting Linkage
The PEG shielding layer and NAG targeting ligand are reversibly linked to the primary amine of PBAVE via pH-sensitive CDM-derived maleic acid bonds. This bond is stable at plasma pH but rapidly hydrolyzes at the acidic pH of the endosome.
Application of DPC-siRNA Conjugates
The therapeutic potential of siRNA dynamic polymer conjugates spans multiple disease areas, and their unique delivery capabilities enable them to target previously difficult-to-drug targets:
 Oncology Applications
Oncology Applications
In cancer treatment, siRNA dynamic polymer conjugates can simultaneously silence multiple oncogenes or drug resistance factors through combined targeting.
 Liver and Metabolic Diseases
Liver and Metabolic Diseases
While GalNAc-siRNA conjugates have been successfully used for liver-targeted delivery, dynamic polymer conjugates exhibit even stronger delivery capabilities through multivalent presentation and prolonged retention time.
 Nervous System Diseases and Rare Diseases
Nervous System Diseases and Rare Diseases
The blood-brain barrier is a major challenge in the treatment of nervous system diseases; however, advanced polymer conjugates with optimized physicochemical properties show promising promise for delivery to the central nervous system.
 Antiviral Therapy
Antiviral Therapy
The rapid development potential of siRNA therapy makes it particularly important in addressing emerging viral threats. Dynamic polymer conjugates can be designed to target highly conserved viral sequences or host factors crucial for viral replication, thereby constructing a barrier against antiviral resistance.
Overview of What Creative Biolabs Can Provide
Creative Biolabs offers comprehensive end-to-end services for developing custom siRNA dynamic polymer conjugates,leveraging our extensive experience in bioconjugation chemistry and oligonucleotide therapies. Our service portfolio includes:
Custom Component Design and Synthesis
- siRNA/Oligonucleotide Synthesis
- Polymer Design
-
Ligand Selection and Synthesis
- A variety of different chemical modifications and fluorescent labels
- Flexible synthesis scale from 0.015 µmol to gt; 10 µmol
- Quality control by analytical HPLC, mass spectrometry
Synthesis of Dynamic Polyconjugates
- Synthesis of PBAVE
- Synthesis of siRNA polycouples
siRNA- Polymer Conjugates Modification
- PEG or NAG molecules
- Carboxydimethylmaleic anhydride (CDM)
- Maleic anhydride derivatives
- Reversible modifications
Characterization of Dynamic Polyconjugates
Our Services Workflow
Phase 1: Sequence Design
We begin with comprehensive computational design of siRNA sequences and conjugate structures. Our proprietary algorithms integrate gene-specific parameters, modification patterns, and structural factors to generate candidate molecules with predicted high activity and specificity.
Phase 2: Synthesis and Conjugation
Our manufacturing capabilities span from research-grade (nanomolar) to clinical-grade (molar) production, with comprehensive analytical validation (HPLC, mass spectrometry, capillary electrophoresis) at each process step.
Phase 3: Formulation and Delivery Optimization
We implement appropriate delivery strategies based on specific applications, including GalNAc conjugation for liver delivery, lipid nanoparticle formulations for tissue-specific targeting, or antibody conjugation for receptor-mediated uptake.
Phase 4: In Vitro and In Vitro Evaluation.
Our in vitro screening includes cell-based potency assays (mRNA and protein level quantification), mechanism of action studies (RISC loading efficiency, subcellular localization), and preliminary safety assessments (cytotoxicity, immunostimulation).
Phase 5: Lead Compound Screening and Scale-up
We facilitate a seamless transition from candidate compound screening to development through structured lead compound optimization and process scale-up.
What Makes Creative Biolabs Your Top Choice
✅Modular Platform: The platform is truly modular, allowing for fast turnaround on switching cargo, ligand, and polymer architecture.
✅Precise Chemistry: We have precise control of the pH-sensitive and reduction-sensitive linkers to ensure maximal stability in vivo and maximum release intracellularly. We ensure a well-defined Drug-to-Polymer Ratio and product homogeneity.
✅Experience with Oligonucleotide Therapeutics: Our vast experience with siRNA, ASO and mRNA conjugation ensures that the therapeutic payload remains fully active after conjugation.
✅Scalability: Services range from small-scale (milligram scale) research synthesis for research production.
Frequently Asked Questions
Q: How do dynamic multimeric siRNA conjugates differ from regular siRNA conjugates?
A: Dynamic multimeric siRNA conjugates are characterized by the incorporation of reversible covalent bonds. These bonds are designed to be stable during cycling but undergo controlled cleavage in response to specific intracellular stimuli (e.g., acidic pH, reducing conditions, enzyme activity, etc.). Compared to regular conjugates, this stimulus-response behavior allows for more precise control of siRNA release, resulting in enhanced therapeutic efficacy and reduced off-target effects.
Q: How do you know multimeric siRNA conjugates are better than regular siRNA conjugates?
A: Recent work has shown tetrameric siRNA conjugates can achieve an impressive liver-kidney distribution ratio of up to 10: 1 compared to only about 1:1 for monomeric conjugates. This increase in targeting efficiency suggests a significant reduction in renal accumulation (with its associated toxicity concerns) and, at the same time, an increase in plasma half-life through evasion of size-based renal clearance pathways.
Q: Are dynamic multimeric siRNA conjugates able to target extrahepatic tissues?
A: Yes. Targeted conjugation strategies, such as antibody-siRNA conjugates (ARCs), have shown very promising results for extrahepatic applications. Recent work with optimally designed ARCs has resulted in 70-80% gene silencing of tumors without cationic transfection reagents. This dramatically increases the number of potential siRNA therapeutic applications to include extrahepatic tissue targeting.
Q: How do you tackle the issue of endosome escape?
A: We utilize many strategies to promote endosome release, including the incorporation of endosome-disrupting peptides (e.g. GALA3, H12, etc.), pH-responsive polymers that change conformation at acidic pH, and optimized surface charge characteristics. We have also found that prolonged intracellular retention time (of up to 4 days) can actually promote slow endosome escape and leads to dramatic improvements in gene silencing efficiency.
Q: What is the time frame on typical projects from lead compound design to lead compound validation?
A: We are set up to have an initial candidate sequence available within 3 days. Synthesis typically requires 4+ days, formulation optimization takes 2+ weeks, and then there is biological evaluation that usually takes 3+ weeks. So, a lead compound should be ready to go within 8-10 weeks of project start, but this is flexible and will change based on the complexity and requirements of the project.
Conslusion
Leveraging our extensive expertise in RNA-related products and services, we are well-prepared to assist you in addressing any challenges that may arise. Our team of experts is fully equipped to cater to your unique requirements. Through our services, we aim to enhance and expedite your drug discovery and disease treatment research initiatives. Don't hesitate to contact us!
Reference
- Tai W. Current aspects of siRNA bioconjugate for in vitro and in vivo delivery. Molecules, 2019, 24(12): 2211. https://doi.org/10.3390/molecules24122211 Distributed under Open Access license CC BY 4.0, without modification.
