Custom Polymer-siRNA Conjugation Service
Creative Biolabs is one of the most innovative and reliable bioconjugation technology suppliers in the field of custom nanoparticle-siRNA conjugation service. The custom bioconjugation platform is being introduced to help you discover the therapeutic potential of gene silencing with controllability and precision that the conjugation process requires. In fact, the RNAi therapeutics we are developing overcomes the most challenges: stability of the system, cell uptake, and endosomal escape.
Polymer-siRNA Conjugate Introduction
siRNA
Small interfering RNA (siRNA) molecules represent a powerful class of nucleic acid therapeutics that sequence-specifically silence target genes through the RNA interference (RNAi) pathway. However, the inherent physicochemical properties of natural siRNAs—particularly their anionic nature, large size, susceptibility to nuclease degradation, and rapid renal clearance—pose significant challenges to their effective systemic delivery in vivo.
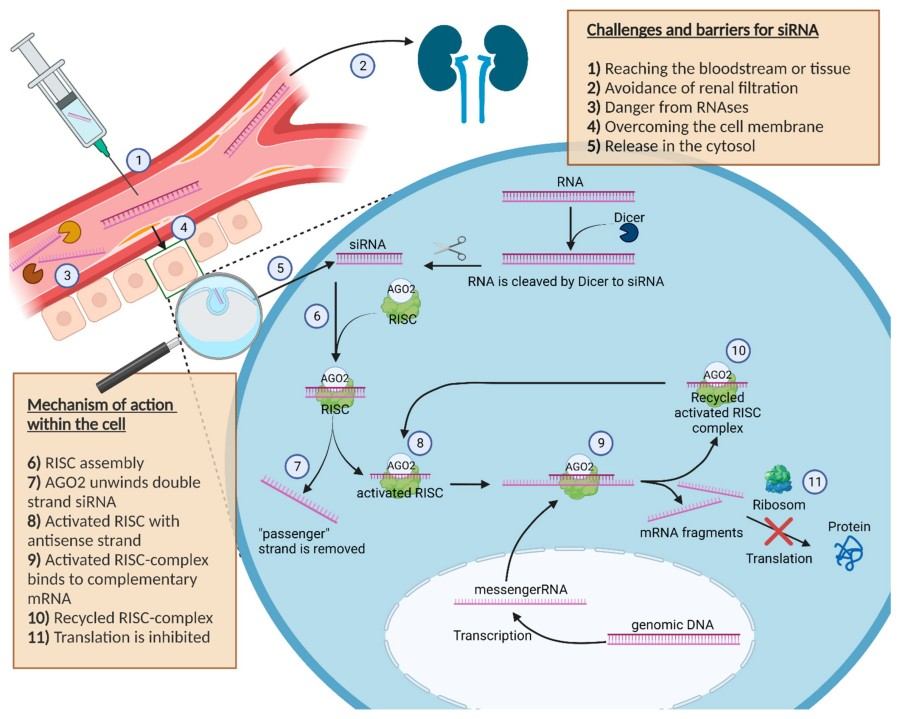 Figure 1 Schematic illustration about the challenges, barriers and the mechanism of action of siRNA in the cell.1
Figure 1 Schematic illustration about the challenges, barriers and the mechanism of action of siRNA in the cell.1
Polymer-siRNA Conjugate
Polymer-siRNA conjugates address these limitations by covalently linking the siRNA payload to a polymer backbone. This creates a new type of chimeric therapeutic entity, typically a polyanion or neutral macromolecule complexed with anionic siRNA. The resulting conjugates are designed to fundamentally alter the pharmacokinetics (PK) of the siRNA, enhance its stability, and facilitate its targeted delivery to diseased tissues. Unlike non-covalent delivery systems such as liposomes or polymeric micelles, covalent linkage ensures a stable drug-to-carrier ratio, eliminates premature payload release, and provides the necessary level of control for clinical translation. The design of these systems is rooted in nanomedicine principles, in which the polymer backbone dictates size, surface charge, and ultimate fate in the biological environment.
Why Use Polymer-siRNA Conjugate?
Naked siRNA duplexes are a prime example of a biologic with high potency but poor drug-like properties. Their systemic administration faces a number of hurdles:
- Nuclease degradation: Serum nucleases rapidly degrade unprotected siRNA, reducing its half-life in the circulation to minutes.
- Renal clearance: siRNA's small size (~13 kDa) and highly anionic phosphate backbone facilitate rapid renal filtration and excretion.
- Immune activation: Certain siRNA sequences can trigger unintended innate immune responses through Toll-like receptors (TLRs).
- Cellular uptake: siRNA's negative charge repels anionic cell membranes, preventing efficient cellular internalization.
- Endosomal retention: Even after internalization, siRNA is primarily trapped in endosomal and lysosomal compartments, where it is degraded without reaching the cytoplasmic RISC.
Linker Design and Precision Conjugation Strategy
Linker Design
A. Removable (Reversible) Linkers
These linkers are designed to be specifically cleaved in response to biological stimuli within the target cell or tissue, ensuring release of free, active siRNA.
- Reduction-Sensitive Disulfide Linkers: These linkers are cleaved by the high concentration of intracellular glutathione (GSH). The GSH concentration in the cytoplasm (≈2–10 mM) is significantly higher than that in the extracellular space (≈2–20 μM). This difference provides an ideal trigger for cytoplasmic release.
- pH-Sensitive Linkers: These utilize acid-labile groups (e.g., hydrazone, acetal bonds) that are rapidly hydrolyzed in the acidic endosomal environment, promoting release prior to lysosomal fusion and degradation.
- Enzyme-Sensitive Linkers: Oligopeptide sequences (e.g., GFLG, which is susceptible to cleavage by cathepsin B) are introduced to allow release only upon exposure to specific lysosomal enzymes, which are often upregulated in certain disease states, such as tumors.
B. Non-cleavable (Stable) Linkers
In some applications, such as oligonucleotide conjugates designed for high stability or specific splicing modulation, a stable linkage (e.g., a simple amide bond formed via carbodiimide chemistry) is preferred, where the siRNA may still be active when covalently attached to a small carrier molecule or when the linkage is formed at a noncritical position.
Precision Conjugation Strategies
- Click chemistry (Cu(I)-catalyzed azide-alkyne cycloaddition - CuAAC): Highly reliable, nearly quantitative, and biorthogonal. Requires functionalization of a polymer with an alkyne and functionalization of the siRNA with an azide group (typically at the 3′ end).
- Strain-promoted azide-alkyne cycloaddition (SPAAC): A copper-free click chemistry variant using strained cyclooctynes, suitable for biological systems to avoid copper toxicity, although generally not as fast as CuAAC.
- Thiolmaleimide coupling: A highly selective reaction between a terminal thiol on the siRNA and a functionalized maleimide on the polymer, forming a stable thioether bond. This is rapid and efficient at physiological pH.
Core Services at Creative Biolabs
Nanoparticle siRNA conjugation is not only an auxiliary step, but also mandatory for achieving the full clinical potential of RNA interference. The complexity of balancing stability, targeting specificity, and controlled intracellular release requires expertise in advanced materials science and bio coupling chemistry. At Creative Biolabs, we provide end-to-end solutions for your polymer-siRNA conjugate needs:
- Polymer Synthesis and Functionalization: Custom synthesis of polymers with controlled molecular weight, polydispersity, and terminal functional groups (azide, alkyne, maleimide, NHS, etc.).
- siRNA Design and Modification: Design and chemically synthesize siRNA duplexes with site-specific modifications (2'-OMe, 2'-F) for stability and reduced immunogenicity, as well as desired conjugation handles (thiol, amine, azide).
- Conjugate Synthesis and Purification: Efficient conjugation is achieved using optimized chemistry, followed by rigorous purification (HPLC, FPLC) and characterization (DLS, MALDI-TOF, gel electrophoresis).
-
Comprehensive In Vitro and In Vivo Validation: We offer a full suite of testing, including:
- ✅Serum stability assays
- ✅Cellular uptake studies (flow cytometry, confocal microscopy)
- ✅Gene silencing efficacy (qPCR, Western blot)
- ✅Cytotoxicity and immunogenicity assays
- ✅Pharmacokinetic and biodistribution studies in animal models.
Types of Polymers for Conjugate
The choice of polymer is a key determinant of the success of a conjugate, influencing its stability, biocompatibility, cellular uptake, and intracellular fate. At Creative Biolabs, we have curated an extensive library of polymers, each with distinct chemical and biological properties. The table below categorizes the major polymer classes we use, detailing their structures, key mechanisms, advantages, and considerations.
| Polymer Class | Primary Mechanism of Action | Advantages |
|---|---|---|
| Polyethylene Glycol (PEG) | Stealth Shielding & PK Modulation: Creates a hydrophilic, steric barrier that reduces opsonization and recognition by the mononuclear phagocyte system (MPS). |
|
| Cationic Polymers | "Proton Sponge" Effect & Condensation: High density of amine groups buffers endosomal acidification, leading to osmotic swelling and rupture. Also condenses siRNA via electrostatic interactions in non-covalent complexes, but we use it for defined covalent conjugates. |
|
| Natural & Biodegradable Polymers | Mucoadhesion & Biocompatibility: Chitosan's cationic nature interacts with anionic cell membranes and mucin. It is naturally degraded by lysozyme. |
|
| Advanced Synthetic Platforms | Modular, Multifunctional Scaffolds: Provides a backbone for the simultaneous attachment of siRNA, targeting ligands, and diagnostic agents. |
|
Our Service Process: From Concept to Functional Nanoparticles
Our service is an end-to-end collaborative partnership:
- Consulting and Design: We work with you to identify target genes, disease pathologies, and ideal routes of administration.
- siRNA Sequence and Modification: We provide bioinformatics analysis to select the optimal siRNA sequence and advise on necessary chemical modifications (e.g., 2'-O-methyl, 2'-fluoro) to enhance nuclease resistance and minimize off-target effects.
- Nanoparticle Synthesis and Conjugation: Our core expertise. We select the optimal nanocarrier platform, synthesize under GMP-like conditions, and execute the selected bioconjugation strategy under strict quality control.
- Comprehensive Characterization: We provide complete analytical reports, including particle size (DLS), zeta potential, conjugation efficiency (HPLC, gel electrophoresis), morphology (TEM), and in vitro functionality (gene silencing assays).
- Optional In Vivo Validation: We offer preclinical testing in relevant animal models to demonstrate biodistribution, pharmacokinetics, and ultimately therapeutic efficacy.
What Makes Our Services the Right Choice?
- World-Class Bioconjugation Expertise: Our team is led by PhD-level scientists with deep practical knowledge in polymer chemistry and nucleic acid biochemistry.
- Proprietary Polymer Library: Access a large library of pre-validated proprietary polymers to enhance delivery capabilities.
- "Smart" Linking Technology: We don't just link; we engineer the release mechanism for maximum intracellular activity.
- Strict Quality Control: Each batch is thoroughly characterized, ensuring you receive the highest quality and consistency.
- Confidentiality and Intellectual Property Protection: Your project details and intellectual property are protected by a strong nondisclosure agreement.
Frequently Asked Questions
Q: What is the typical molecular weight range for the final polymer-siRNA conjugate?
A: The molecular weight is highly dependent on the chosen polymer backbone (e.g., PEG, HPMA) and the polymer-to-siRNA ratio. Typically, the final conjugate is designed to be well above the renal filtration threshold, typically between 40 kDa and 150 kDa, to ensure prolonged systemic circulation and passive tumor accumulation via the EPR effect.
Q: How do you confirm the integrity and activity of the conjugated siRNA?
A: We use a variety of orthogonal methods. Denaturing polyacrylamide gel electrophoresis (PAGE) confirms covalent attachment and integrity (absence of degradation fragments). Functional activity is confirmed by standard gene silencing assays (in vitro knockdown of a reporter gene) after cleavage (if a cleavable linker is used) to ensure that the released siRNA retains full biological potency.
Conclusion
Custom polymer-siRNA conjugation services are a key enabling technology for realizing the full therapeutic potential of RNA interference (RNAi). Free siRNA is biologically unstable, easily degraded by nucleases, and rapidly cleared by the kidneys. By covalently linking siRNA to precision-engineered polymer backbones (such as PEG, HPMA, or PBAE) via custom cleavable or stable linkers, Creative Biolabs creates stable, complex chimeric molecules. If you are interested in our biojugation services, please feel free to contact us for more details.
Reference
- Gabel M, Knauss A, Fischer D, et al. Surface design options in polymer-and lipid-based siRNA nanoparticles using antibodies. International Journal of Molecular Sciences, 2022, 23(22): 13929. https://doi.org/10.3390/ijms232213929. Distributed under Open Access license CC BY 4.0, without modification.
