Custom Small Molecule-Antibiotic Conjugation Service
What is Small Molecule-Antibiotic Conjugation?
Small molecule antibiotic coupling is the chemical coupling of antibiotics with small molecules that possess the desired properties. The functions of small organism coupling molecules include regulating the pharmacokinetics of drugs, enhancing cell wall permeability, and increasing the targeting of coupled antibiotics to specific bacteria. Bioconjugation methods are the processes through which covalent bonds between small molecular weight compounds and an antibiotic are formed through reaction. Formation of amide bonds, disulfide bonds, and even hydrazone bonds, are all examples of some couplings that are done in bioconjugation. For example, attachment using covalent bonds of surface receptor-binding ligands will allow passage through the bacterial membranes, leading to improved delivery of the targeted antibiotic directly inside the bacteria where it can work best with minimum energy expenditure.
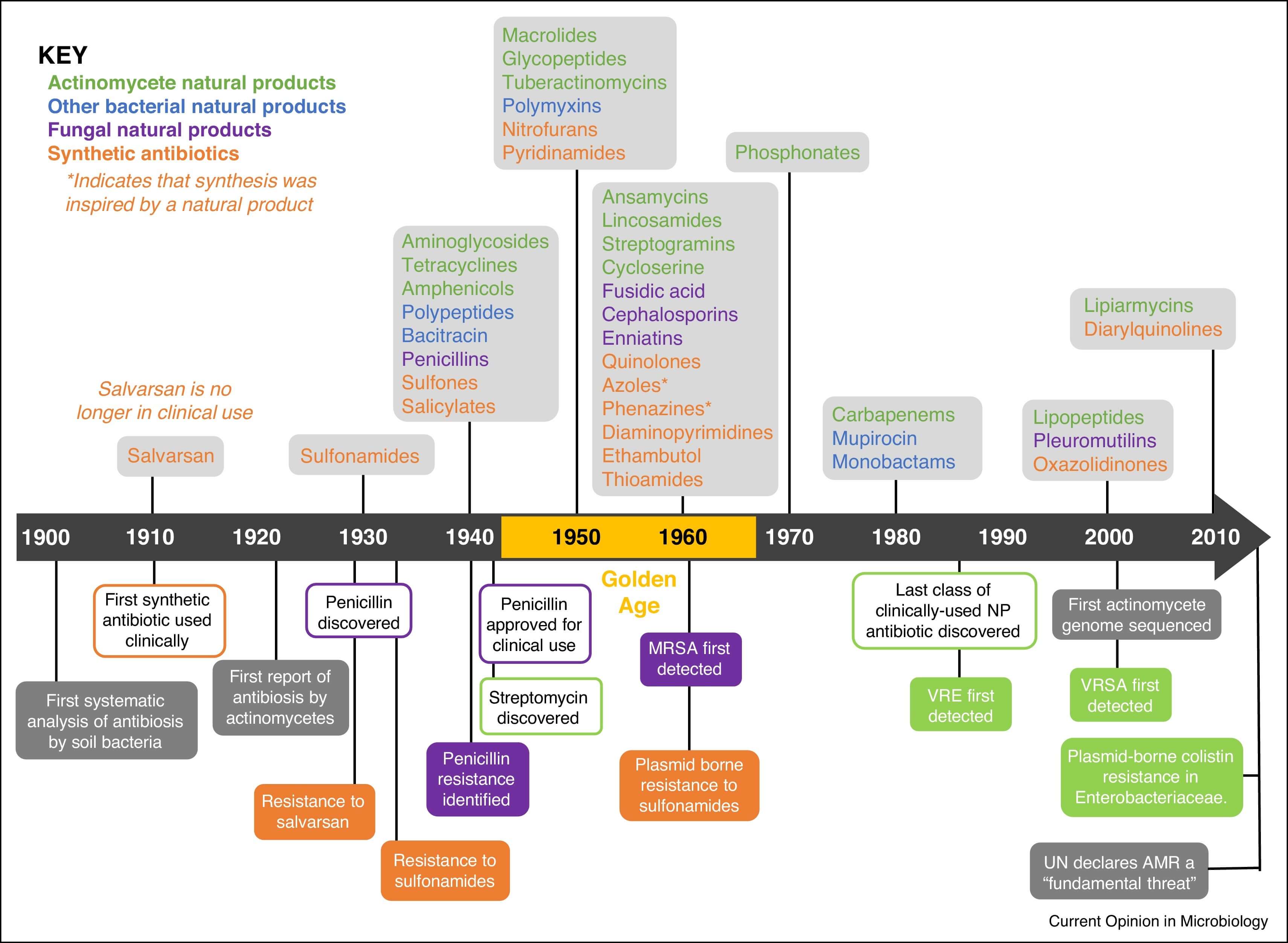 Figure 1 Timeline showing the decade new classes of antibiotic.1,3
Figure 1 Timeline showing the decade new classes of antibiotic.1,3
Key Antibiotic Classes and Mechanisms
| Antibiotic Class | Mechanism of Action | Examples |
|---|---|---|
| β-Lactams | Inhibit peptidoglycan cross-linking | Cephalosporins, Penicillins |
| Aminoglycosides | Bind 30S ribosomal subunit, impair translation | Gentamicin, Streptomycin |
| Macrolides | Block ribosomal peptide elongation | Erythromycin, Azithromycin |
| Fluoroquinolones | Inhibit DNA topoisomerases, disrupt replication | Ciprofloxacin, Levofloxacin |
How Antibiotic Affect Conjugation?
Antibiotics are known to have a variety of effects on conjugation. In some cases sub-lethal levels of some antibiotics have been shown to promote conjugation either by activation of gene transfer or induction of the conjugation machinery. Antibiotics may also have inhibitory effects on conjugation. This can be the case when they target proteins directly involved with conjugation or when they are present at higher and/or lethal concentrations.
- The antibiotic should have accessible reactive groups for conjugation (amines, carboxyls, thiols, etc.) or it can be derivatized.
- Some β-Lactams are sensitive to the conditions used in conjugation (e.g., base) and may require the use of mild reaction conditions to prevent antibiotic inactivation by ring-opening and increase drug penetration.
- An antibiotic targeting DNA gyrase could be paired with a cell wall disrupting agent in a SMAC for dual action.
- Antibiotics can also influence the growth rates of different bacterial populations, which can indirectly affect conjugation.
Which Bacteria have Become Antibiotic Resistant through Conjugation?
| Bacterium | Type of Antibiotic Resistance | Notes |
|---|---|---|
| Enterobacteriaceae (e.g., E. coli, Klebsiella spp., Serratia, Proteus) | Carbapenem-resistant (e.g., through pOXA-48 plasmid), ESBL-producing (Extended-spectrum beta-lactamase), Tetracycline, Chloramphenicol, Quinolones, Penicillins, Cephalosporins, Aztreonam | This group is particularly worrisome because of the wide distribution of resistance genes, particularly carbapenem resistance, a "last-resort" antibiotic. |
| Staphylococcus aureus | Methicillin-resistant (MRSA), Vancomycin-intermediate and resistant (VISA, VRSA), Macrolides, Streptogramins, Fluoroquinolones, Penicillin | MRSA is the poster child of resistance dissemination. Initial resistance may occur through mutation, but dissemination of this resistance on plasmids can be via conjugation. |
| Enterococcus faecium / Enterococcus faecalis | Vancomycin-resistant (VRE) | VRE is a major nosocomial pathogen, with resistance commonly mediated by transmissible plasmids. |
| Pseudomonas aeruginosa | Carbapenem-resistant, Aminoglycosides | An important pathogen in particular in hospital-acquired infections, notorious for picking up multi-drug resistance. |
| Salmonella enterica serovar Typhimurium | Streptomycin, Sulfonamides, Fluoroquinolones | It Can establish persister reservoirs and exchange resistance genes with other gut microbes. |
| Neisseria gonorrhoeae | Cephalosporin-resistant, Fluoroquinolone-resistant, Penicillin | A major public health concern due to increasing resistance, making treatment challenging. |
| Campylobacter spp. | Fluoroquinolone-resistant | Common cause of foodborne illness. |
How can Bioconjugation Contribute to Antibiotic Resistance in Microorganisms?
The nature of bioconjugation does not naturally cause resistance to antibiotics in microorganisms. The possibility of resistance emergence hinges on gene transfer. Bacteria may acquire such genes if present on the small molecules or on antibiotic partners used in the conjugation, rather than intended, or if these genes are contaminants during the conjugation reaction. For instance, if the resistance genes were present in the bioconjugate or reaction medium, bacteria would have a relatively easy time taking them up and developing resistance to antibiotics.
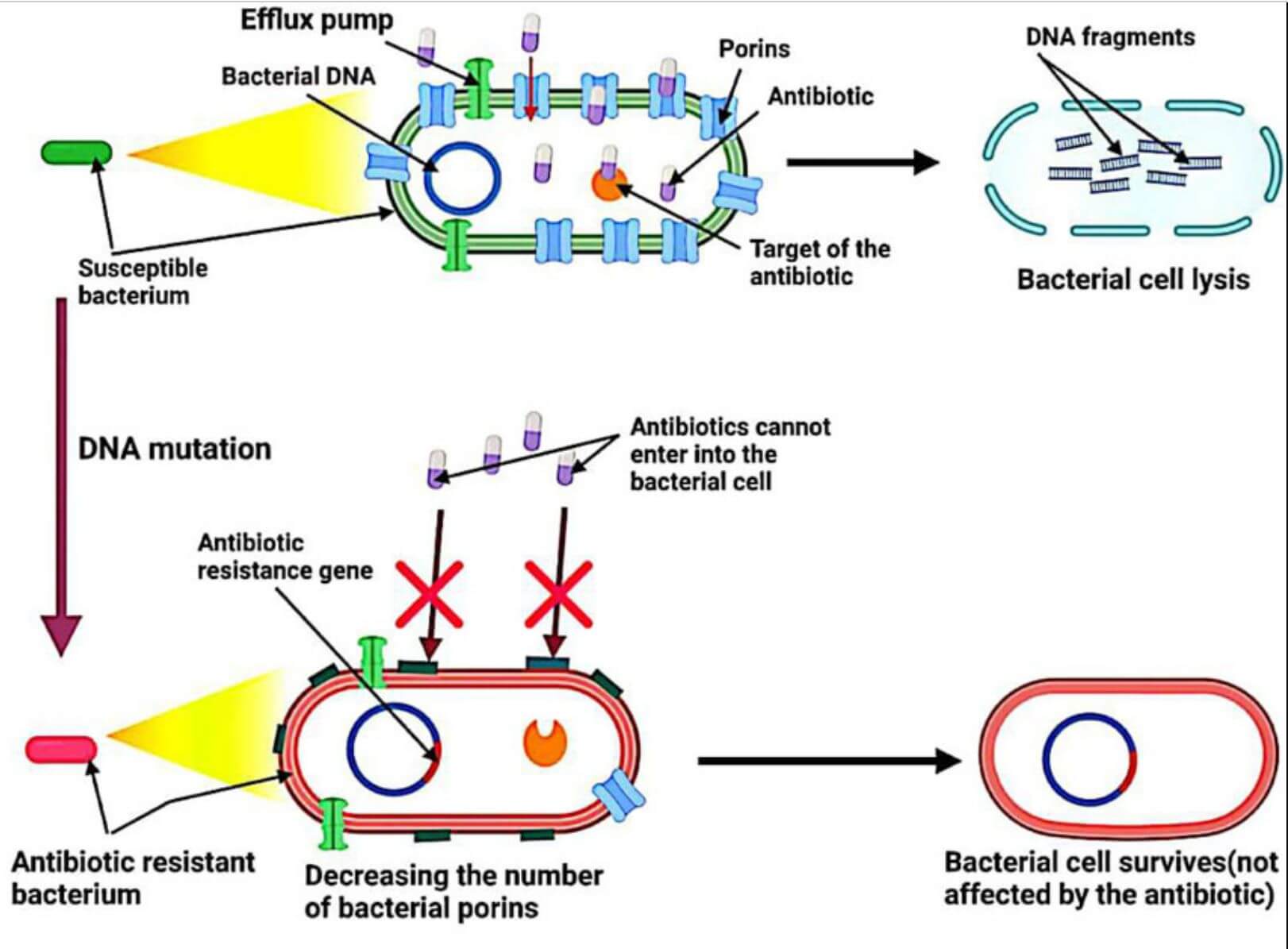 Figure 2 Mechanism of antibiotic resistance.2,3
Figure 2 Mechanism of antibiotic resistance.2,3
Advantages of Small Molecule-Antibiotic Conjugation
Conjugation technology offers innovative solutions to antibiotic resistance:
- Some large antibody conjugates still face several limitations, such as long blood circulation time, low penetration into solid tumors, high development and synthesis costs, and potential immunogenicity. Small molecules as homing devices are promising alternatives to overcome these challenges due to their natural non-immunogenicity, in vitro and in vivo stability, low molecular weight, and overall more cost-effective synthesis and development.
- Combining small molecule modulators with anti-biotin can synergistically enhance the inhibitory effect on tumor progression by enhancing the immune system to fine-tune responses within the TME, strengthening its ability to recognize and eliminate cancer cells.
- Conjugated antibiotics have longer half-life, higher stability, fewer side effects, higher solubility, lower immunogenicity and more precise targeting.
- Small molecule drugs are easy to administer and can usually be taken orally, which significantly improves patient compliance and convenience, making them more suitable for daily use than larger biologics that usually require injection or infusion.
Challenges of Small Molecule-Antibiotic Conjugation
Although small molecule drugs have achieved encouraging results, there are also many challenges. Irrational use of drugs will increase drug side effects and drug resistance, resulting in poor treatment effects. Secondly, with small molecule drugs alone, especially protease inhibitors, cancer cells are prone to drug-resistant mutations in about 2 weeks, and small molecule drugs are prone to produce multiple drug-resistant sites. In short, small molecule drugs are in the ascendant, and new drugs are constantly emerging, making anti-cancer and anti-tumor drug treatment crucial. With the continuous development of such drugs with low drug resistance, high efficacy and few side effects, I believe that in the near future, there will be major breakthroughs in the treatment of cancer.
Small Molecule-Antibiotic Conjugation Services at Creative Biolabs
At Creative Biolabs, our expertise in conjugate design and synthesis enables you to leverage these benefits and advance your antibiotic innovation initiatives with confidence. Our services range from innovative customized design and production for your specific bioconjugation needs. We are well-versed in the complete bioconjugation workflow and characterization of such conjugates for quality, stability and potency.
Recommended services
-
Custom Small Molecule Synthesis
-
Antibiotic Modification and Derivatization
-
Linker Design and Synthesis
Recommended products
Creative Biolabs recognizes the distinct nature of each discovery project. That's why our personalized consultation service is designed to optimize conjugation strategies precisely for your molecules, targets, and research objectives. From straightforward linker attachments to sophisticated multifunctional conjugates carrying multiple payloads, we tailor our approach to fit your exact requirements. If you are interested in our service, please feel free to contact us for more details.
References
- Hutchings M I, Truman A W, Wilkinson B. Antibiotics: past, present and future. Current opinion in microbiology, 2019, 51: 72-80. https://doi.org/10.1016/j.mib.2019.10.008
- Halawa E M, Fadel M, Al-Rabia M W, et al. Antibiotic action and resistance: updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Frontiers in Pharmacology, 2024, 14: 1305294. PMCID: PMC8263657.
- Distributed under Open Access license CC BY 4.0, without modification.
