Inverse Electron Demand Diels-Alder Reaction (IEDDA)
What is Inverse Electron Demand Diels-Alder Reaction (IEDDA)?
IEDDA is the term for a cycloaddition that takes place between electron-deficient dienes and electron-rich dienophiles. The electron-deficient dienes often contain heteroatoms, and the resulting products are six-membered rings, making this reaction particularly valuable in the chemical synthesis of heterocyclic natural products. The tetrazine ligation was first reported independently by two groups in 2008, marking the beginning of research and exploration into IEDDA as a bioorthogonal coupling reaction. IEDDA is recognized as the fastest known bioorthogonal reaction to date.
Mechanism of CuAAC
The initial step of the IEDDA involves a [4+2] cycloaddition between a diene and a dienophile. The resulting endocyclic compound with high ring tension rapidly undergoes the elimination of a nitrogen molecule, yielding 4,5-dihydropyrazine derivatives. Thereafter, 1,4-dihydropyrazine isomers are created by an intramolecular proton transfer, which is followed by pyridazine production through oxidation.
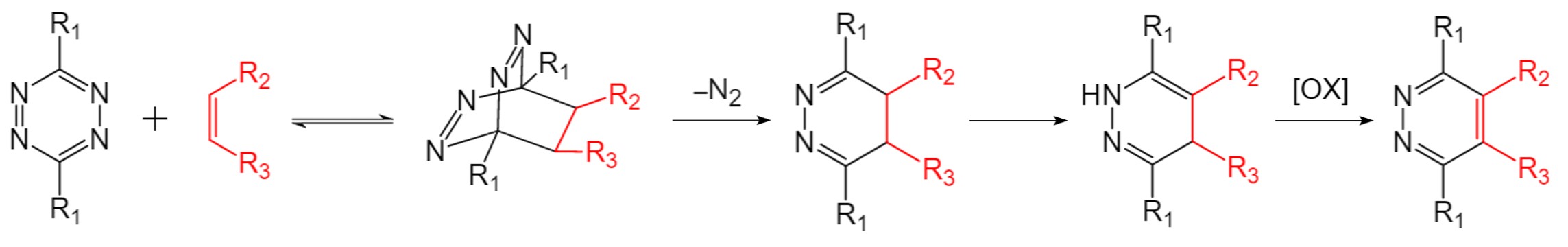 Fig.1 The mechanism of IEDDA reaction.
Fig.1 The mechanism of IEDDA reaction.
Advantages of IEDDA
- The reaction demonstrates excellent biocompatibility, enabling it to occur in aqueous solutions, organic solvents, or biological media.
- IEDDA exhibits high chemical selectivity, allowing reactants to conjugate with biomolecules at very low concentrations.
- The reaction has a fast reaction rate and does not require catalyst activation.
Applications of IEDDA
 Biological Imaging
Biological Imaging
The substrates for the IEDDA reaction are highly modifiable, allowing researchers to introduce chromophores or directional groups at the 3 and 6 positions of the tetrazine by chemical synthesis. This allows for targeted imaging of specific components or regions within biological organisms. The IEDDA reaction facilitates the labeling of fundamental biomacromolecules (e.g., proteins, nucleic acids, and lipids). Furthermore, it enables imaging of common cellular organelles, including mitochondria, lysosomes, and the endoplasmic reticulum, and can even serve as a positron emission tomography (PET) probe for live imaging.
 Targeted Transport of Drugs
Targeted Transport of Drugs
The IEDDA has gained significant traction in cancer therapy due to its rapid reaction rates in various bioorthogonal reactions. Researchers can leverage this technique to conjugate potent tumor-killing drugs onto tetrazine scaffolds, facilitating targeted drug delivery through swift and specific IEDDA conjugation. Additionally, another innovative approach involves linking drug-loaded nanocarriers to antibodies that recognize tumor-specific antigens via IEDDA reactions. This antigen-antibody interaction allows for the transportation of the drug carriers, such as liposomes, directly to tumor sites. Once there, the unique characteristics of the tumor microenvironment, such as pH variations, can be exploited to degrade the drug carriers, thereby achieving targeted drug delivery and release.
 Drug Release
Drug Release
In addition to serving as a drug-targeting agent, researchers can employ IEDDA reaction for site-specific release of drugs. Rossin et al. successfully developed an antibody-drug conjugate (ADC) linked via trans-cyclooctene (TCO) in their study.1 This ADC binds to non-internalizing cancer cell receptors and subsequently undergoes a click reaction with a tetrazine-derived activator through TCO. Since the allylic carbamates are cleaved from TCO, this mechanism facilitates the intracellular release of the drug, leading to the development of bioorthogonal "click-to-release" applications for both in vitro and in vivo settings.
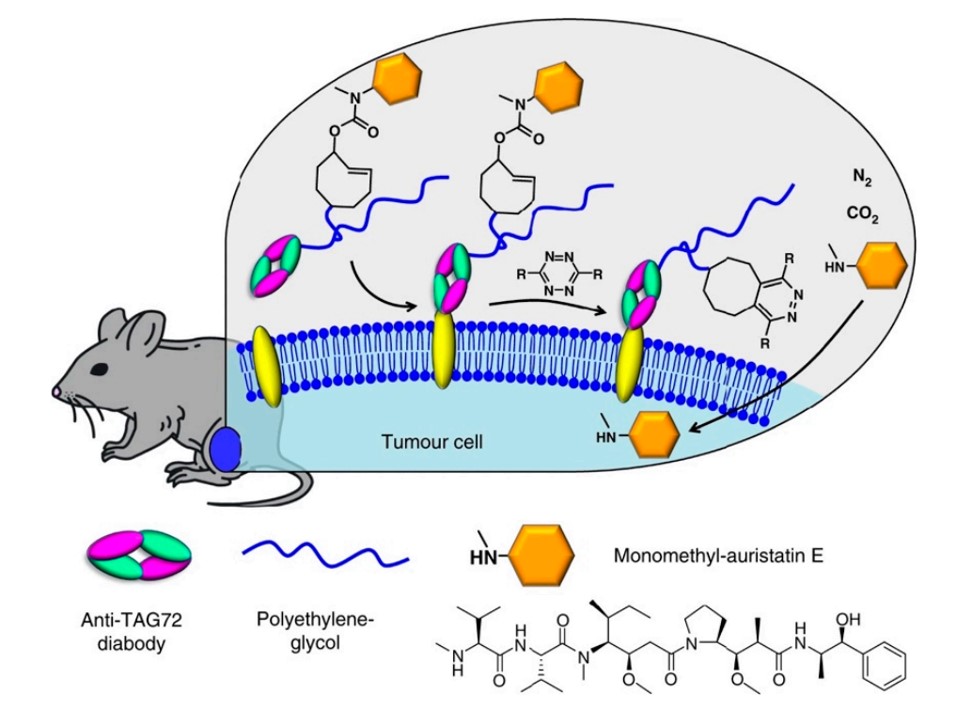 Fig.2 Triggering drug release by using "click-to-release" chemistry in vivo.1
Fig.2 Triggering drug release by using "click-to-release" chemistry in vivo.1
Reference
- Rossin, Raffaella, et al. "Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice." Nature communications 9.1 (2018): 1484. Distributed under Open Access license CC BY 4.0, without modification.
Related products
