Custom Small Molecule-Drug Conjugation Service
What Is a Small Molecule Drug Conjugate?
A new option for targeted therapy is the development of new type of "lightweight" drugs based on the ADC design idea, which are called small-molecule drug conjugates (SMDCs). SMDCs are the products of the conjugation of small-molecule targeting ligands with cytotoxic drugs. They have three main parts, namely small-molecule targeting ligands, cytotoxic molecules and linkers. SMDCs have many advantages compared with ADCs, including more rapid and even dispersion into tumor tissues, low cost and no immunogenicity. SMDCs take advantage of a small molecule, like a vitamin, a hormone or a receptor ligand, to home in on an overexpressed receptor that is present on the surface of cancer cells, allowing for preferential release of the highly toxic payload in the tumor microenvironment.
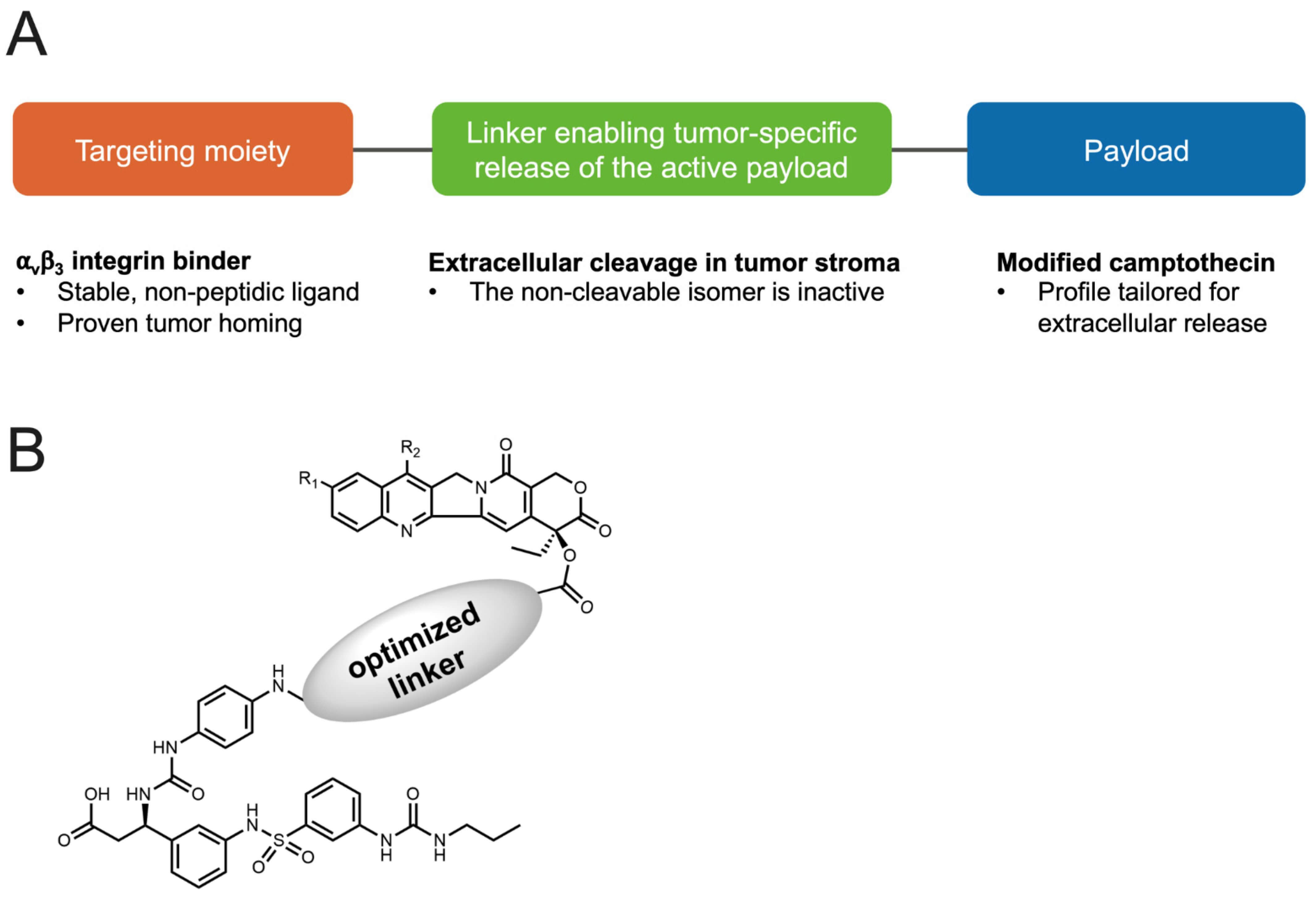 Figure 1 Structure of the small molecule–drug conjugate VIP236.1
Figure 1 Structure of the small molecule–drug conjugate VIP236.1
How Do Small Molecule-Drug Conjugates Work?
The mechanism of action of SMDC is a series of highly coordinated events aimed at targeted cell killing.
- Targeted Binding: When the small molecule targeting ligand of SMDC selectively binds to its corresponding receptor, the process begins and the receptor is overexpressed on the surface of the target cancer cell.
- Internalization: After binding, the receptor SMDC complex is internalized by the cell through a process called receptor-mediated endocytosis. The entire complex is enveloped by the cell membrane and transported into the endosome.
- Trafficking and Linker Cleavage: The endosome is trafficked to lysosomes. Lysosomes are acidic and rich in enzymes. They are cell compartments where the low pH value or presence of specific enzymes (e.g. protease B) can cause the linker to be cleaved, thus releasing a highly active cytotoxic payload.
- Payload Action: The active payload can diffuse from the lysosome into the cytosol where it can reach its molecular target to elicit its intended effect. For example, microtubule inhibitors prevent the normal assembly of the cell cytoskeleton and lead to cell cycle arrest and programmed cell death (apoptosis).
Antibody Drug Conjugates vs. Small Molecule Drug Conjugates
| Feature | Small Molecule-Drug Conjugates (SMDCs) | Antibody-Drug Conjugates (ADCs) |
|---|---|---|
| Size | Small (~1 kDa) | Large (~150 kDa) |
| Tumor Penetration | Excellent. Can penetrate dense tumor tissue. | Limited. Penetration into solid tumors can be poor. |
| Immunogenicity | Generally low to none. | Potential for anti-drug antibody (ADA) response. |
| Receptor Internalization | Can be highly efficient. | Highly dependent on the specific antibody-antigen interaction. |
| Manufacturing | Easier and more cost-effective. | Complex and expensive. |
| Pharmacokinetics | Rapid clearance, short half-life. | Slower clearance, longer half-life. |
| Challenges | Off-target toxicity if receptor is present on healthy cells. Potential for premature payload release. | Immunogenicity. Off-target toxicity if antigen is present on healthy cells. |
Small Molecule Drug Conjugate Design
The successful design of SMDC is a multifaceted process that requires careful consideration of each of its three components to ensure optimal therapeutic outcomes. The goal is to achieve a balance between cyclic stability and effective release of the target site.
Small Molecule Targeted Ligands
The targeted ligands of SMDC play a crucial role in selectively delivering therapeutic payloads to targeted macromolecules. As for safety issues, it depends on the biological distribution and target location of SMDC binding. Therefore, the correct selection of tumor cell targets and corresponding ligands is crucial for reducing the toxicity of conjugates. In terms of effective design of SMDCs, scientists at Creative Biolabs have fully considered the characteristics of SMDCs, including binding affinity, targeting specificity, and drug conjugate size. We can provide targeted ligand selection services for existing and undeveloped receptors. Our SMDC receptor selection includes:
- Folic acid receptor (FR)
- Prostate specific membrane antigen (PSMA)
- Somatostatin receptor (SSTR)
- Carbonic Anhydrase IX (CAIX)
- Phosphatidylserine receptor
Cytotoxic Payload
Choosing cytotoxic drugs or payloads is due to their high efficacy. These payloads are usually too toxic to be administered systemically alone. Frequent use of microtubule inhibitors or DNA damaging agents as effective payloads. The ideal payload should have an effective mechanism of action against the target cancer cell type and be suitable for chemical binding without losing its cytotoxic activity.
Linker
A linker is a bridge between small molecule and payloads. The design of linker is a balance between stability and release. Connector should be stable in the blood stream, otherwise it will release its payload and cause systemic toxicity. It should be cleavable in target cell or tumor microenvironment so that it can release active payloads to play its cytotoxic roles.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a global leader in SMDC development, providing customized integrated services to accelerate the transition of SMDC from early research to clinical trials. Our team of bio coupling experts, pharmaceutical chemists, and preclinical scientists provides tailored solutions for every step of SMDC development. If you are interested in our bioconjugation services, please feel free to contact us for more details.
SMDC Design and Optimization
- Target selection and validation: bioinformatics analysis and experimental confirmation of target overexpression in disease tissues
- Ligand screening and optimization: high-throughput screening and medicinal chemistry optimization of small molecule targeted ligands
- Connector design and evaluation: customized synthesis of cuttable and uncut connectors, and comprehensive stability assessment
- Payload selection and modification: Access a large library of potent cytotoxic drugs and possess expertise in payload modification for conjugation
Linker-Payload Conjugation
- Linker Customization: Design and synthesize cleavable (peptides, disulfides, hydrazones) and non-cleavable (amides, amino esters) connectors with optimized stability and release kinetics.
- Payload Integration: using site-specific chemistry (such as click chemistry, maleimide thiol coupling) in combination with cytotoxic drugs (such as immunostimulants or radioactive isotopes (177Lu, 90Y).
Indications of Our SMDCs
Compared to traditional non targeted therapies, SMDC reduces accidental toxicity by selectively delivering therapeutic payloads to target cells, thereby limiting exposure to healthy cells. SMDC can typically identify overexpressed targeted receptors in diseased tissues based on imaging of the same targeting ligands associated with radioactive tracers. SMDCs provide greater space for drug discovery and optimization, as their safety and efficacy are structurally clear due to the independent variable parts of the molecules. SMDC can also be used for other additional purposes, including accelerating optimal tumor resection and using ligand targeted near-infrared dyes to isolate malignant cancer cells from the blood during surgery. Other applications of SMDC have emerged, such as ligand targeted drugs used in clinical applications to treat various cancers. Our SMDC development service supports the following applications:
- Ovarian cancer
- Prostate cancer
- Neuroendocrine tumors
- Liver cancer
- Various solid tumors
- Inflammatory diseases
- Infectious diseases
- Neurological disorders
- Fibrotic conditions
Reference
- Lerchen H G, Stelte-Ludwig B, Kopitz C, et al. A small molecule–drug conjugate (SMDC) consisting of a modified camptothecin payload linked to an αVß3 binder for the treatment of multiple cancer types. Cancers, 2022, 14(2): 391.https://doi.org/10.3390/cancers14020391 Distributed under Open Access license CC BY 4.0, without modification.
