Custom Oligonucleotide-Aptamer Conjugation Service
Bioconjugation refers to the technique of linking two biologically related molecules through covalent bonds. The application objects of this methodology can be roughly divided into two categories: targeting non-natural functional groups and targeting native functional groups in organisms. Although the latter has an overwhelmingly wide range of applications, the development difficulty is also very high from the perspectives of functional group selectivity and reaction controllability. In addition, strict conditions such as suitability for biological conditions (neutral pH, room temperature, aqueous reactions, etc.), pollution-free reagents, waste, etc. are also required. Therefore, the reactions developed so far still have significant room for improvement. Creative Biolabs is an important company in biotechnology. It leads in this area of innovation. The company provides advanced Oligonucleotide-Aptamer Bioconjugation services and bioconjugation technology. These services are designed to meet the varied needs of academic and industrial sectors.
Introduction to Oligonucleotides
Oligonucleotides are a type of short chain nucleic acid composed of deoxyribonucleotides or ribonucleotides. They can be paired with DNA, mRNA, or pre mRNA based on the principle of complementary base pairing, accurately "silencing" certain genes and preventing the expression of many proteins. Common oligonucleotides are antisense oligonucleotides (ASO), small interfering RNA (siRNA), microRNA, and aptamers. The main obstacles to developing oligonucleotide drugs are insufficient pharmacokinetic properties and poor cellular uptake. The covalent linkage of multiple ligands aimed at influencing biological distribution and cellular uptake or targeting specific tissues is a highly attractive possibility for advancing therapeutic applications and expanding development strategies. Compared to advanced formulations typically composed of multiple reagents and sensitive to various preparation conditions, oligonucleotide conjugates are a well-defined molecule that enables structure-based analysis and quality control techniques.
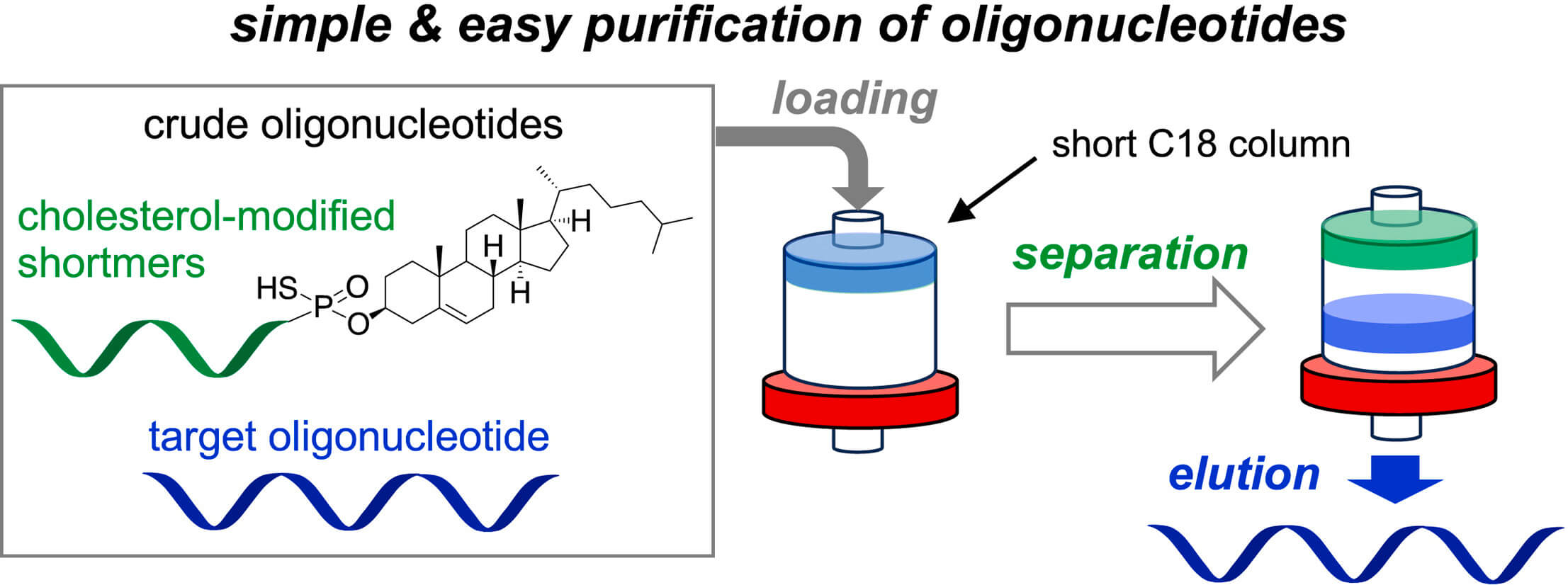 Figure 1 Development of simple purification method for oligonucleotides.1,3
Figure 1 Development of simple purification method for oligonucleotides.1,3
What are Aptamers?
The adapter is a short nucleic acid oligonucleotide. They can be used as both drugs and drug carriers. Their use as diagnostic tools is also evident. They can be generated using various experimental, theoretical, and computational techniques. Systematic evolution of ligands through index enrichment (iterative screening using nucleic acid libraries) is a popular experimental technique. Another approach is based on theoretical inspiration - entropy based seed and growth strategies, which design adapter templates to specifically bind to targets. Aptitudes are predicted to be very useful in the production of common and therapeutic drugs, occasionally used for certain diseases such as cancer, Alzheimer's disease, etc. They bind to various targets such as lipids, nucleic acids, proteins, small organic compounds, and even the entire organism. Key advantages of aptamers include:
- High affinity and specificity
- Small size
- Chemical stability
- Low immunogenicity
- Cost-effectiveness
- Ease of modification
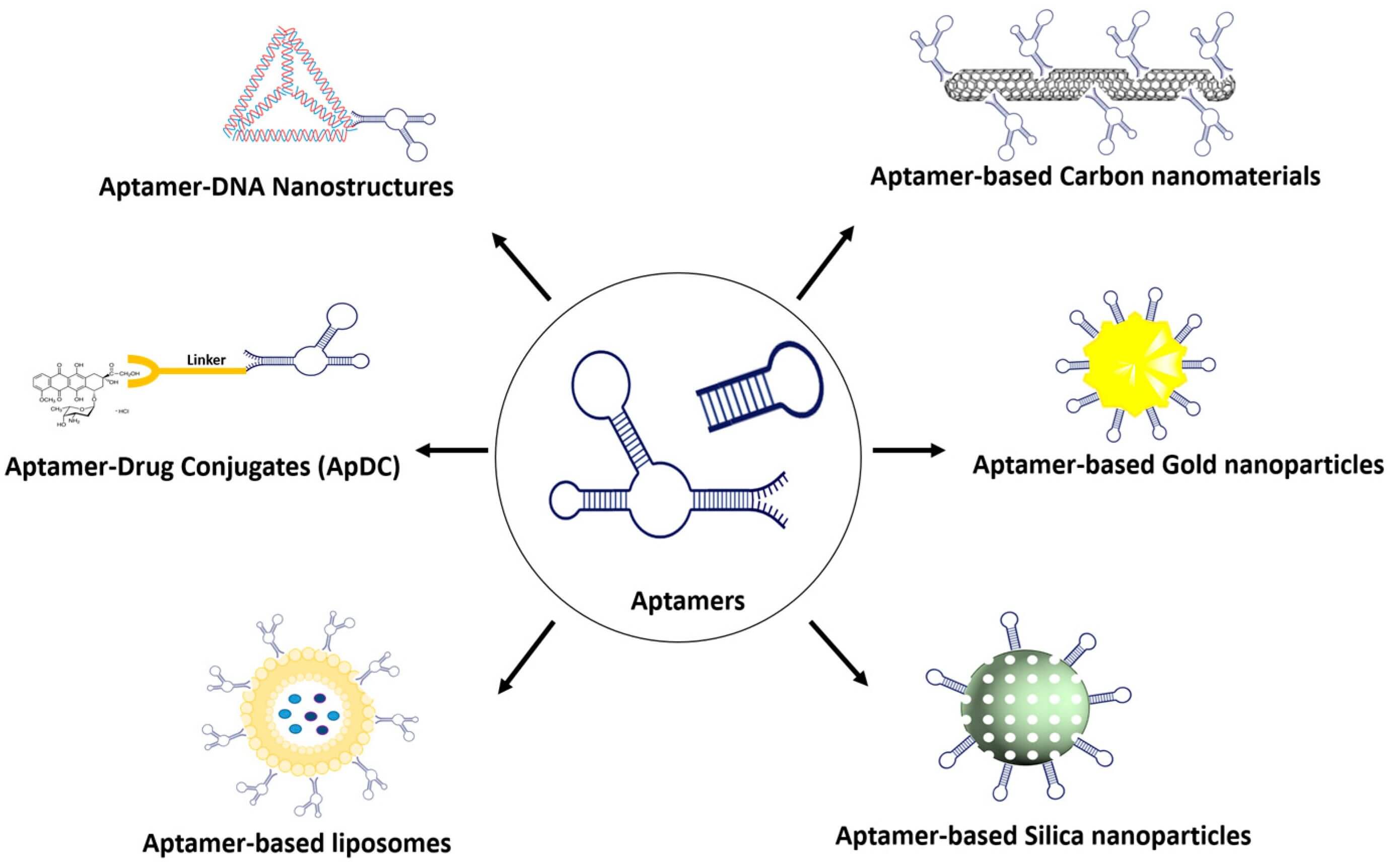 Figure 2 A variety of aptamer conjugates for targeted drug-delivery system.2,3
Figure 2 A variety of aptamer conjugates for targeted drug-delivery system.2,3
Scientific Principles in Oligonucleotide-Aptamer Bioconjugation
Adaptive oligonucleotide coupling provides a simple, effective model. This model allows direct non-covalent or covalent coupling of aptamer sequences with therapeutic agents. pegaptanib serves as an example. It is an L-RNA aptamer made of non-natural L-ribonucleic acid. Pegaptanib shows high affinity and specificity for CXCL12. It blocks CXCL12 binding to receptors CXCR4 and CXCR7. Thus, pegaptanib treats hematological malignancies like multiple myeloma and acute myeloid leukemia. It also treats solid tumors. Target molecule binding induces aptamer conformational changes. These changes form structures including hairpins, helices, stem-loops, or G-quadruplexes.
-
Covalent Conjugation
This involves forming a stable chemical bond between the aptamer and the molecule or material. Common methods include using cross-linkers (like EDC/NHS for amine-to-carboxyl coupling) or click chemistry.
-
Non-Covalent Conjugation
This relies on weaker interactions like electrostatic forces, hydrogen bonding, or hydrophobic interactions. Examples include biotin-streptavidin interactions and aptamer adsorption onto surfaces.
Linker Selection in Oligonucleotide-Aptamer Bioconjugation
The choice of linker is as crucial as the conjugation chemistry itself. Linker properties are summarized below:
| Property | Description |
|---|---|
| Cleavable vs. Non-cleavable | Cleavable linkers (e.g., pH-sensitive, enzyme-cleavable, disulfide-reducible) allow for controlled release of the oligonucleotide payload inside target cells. Non-cleavable linkers provide stable, permanent conjugates. |
| Length and Flexibility | Influence the ability of the conjugated molecules to interact with their targets without steric hindrance. |
| Hydrophilicity/Hydrophobicity | Can impact solubility and in vivo behavior of the conjugate. |
Creative Biolabs' Services
Creative Biolabs is a leader in oligonucleotide aptamer bioconjugation and offers comprehensive customized services to address unique customer requirements. Our expert scientific team employs the Systematic Evolution of Ligands by Exponential Enrichment method. This technique screens single-stranded DNA or RNA libraries to identify suitable aptamers. We produce high-purity conjugates with custom designs. Our services cover all stages from initial oligonucleotide and aptamer design to final coupling and purification. We optimize every process step to ensure maximum yield and purity. This approach guarantees high biological activity in all oligonucleotide aptamer bioconjugates. Our services include but are not limited to:
-
Custom Aptamer Synthesis
-
High-Purity Oligonucleotide Synthesis
-
Diverse Bioconjugation Chemistries
-
Expert Linker Design and Synthesis
Our Methods
Creative Biolabs employs a comprehensive suite of advanced bioconjugation chemistries:
-
Amine-Reactive Chemistry (NHS Esters)
- Principle: N-hydroxysuccinimide (NHS) esters react readily with primary amines (e.g., lysine residues in peptide aptamers, or amine-modified oligonucleotides) to form stable amide bonds.
- Application: Widely used due to its high efficiency and mild reaction conditions. Amine groups can be readily introduced onto oligonucleotides at the 5' or 3' termini via phosphoramidite chemistry.
-
Thiol-Reactive Chemistry (Maleimides)
- Principle: Maleimide groups react selectively and rapidly with free sulfhydryl (thiol, -SH) groups via Michael addition to form stable thioether linkages.
- Application: Cysteine residues (in peptide aptamers) or thiol-modified oligonucleotides provide excellent sites for highly specific conjugation, minimizing side reactions.
-
Click Chemistry (Copper-Catalyzed Azide-Alkyne Cycloaddition - CuAAC)
- Principle: A robust and highly efficient bioorthogonal reaction between an azide and an alkyne to form a stable 1,2,3-triazole ring. Catalyzed by copper(I).
- Application: Ideal for complex bioconjugates where precise control and minimal interference with biological function are critical.
-
Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) / Copper-Free Click Chemistry
- Principle: Utilizes strained cyclooctynes that react with azides without the need for a copper catalyst.
- Application: Preferred for applications where cellular viability and biocompatibility are paramount.
-
Carbodiimide Coupling (EDC/NHS)
- Principle: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) mediates the formation of amide bonds between carboxylate groups and primary amines. NHS is often used as an additive to improve efficiency and stability.
- Application: Useful for conjugating molecules with available carboxyl and amine groups, often employed for conjugating aptamers to carboxyl-modified surfaces or oligonucleotides.
-
Disulfide Bond Formation and Reduction
- Principle: Formation of a disulfide bond between two thiols, or reductive cleavage of existing disulfide bonds followed by alkylation.
- Application: While less common for direct oligonucleotide-aptamer conjugation, it can be used for reversible linkages or as part of a larger multi-step conjugation strategy.
Conclusion
Oligonucleotide-aptamer bioconjugation marks significant progress in biomedical research. It creates new possibilities for making new treatments and diagnostic tools. Creative Biolabs has strong skills in bioconjugation methods and focuses on aptamer drug development. Creative Biolabs has strong skills in bioconjugation methods and focuses on aptamer drug development. Creative Biolabs provides high-quality services and products to advance biomedical discoveries. If you are interested in our biojugation services, please feel free to contact us for more details.
References
- Ren Q, Osawa T, Obika S. Development of simple purification method for oligonucleotides synthesized using phosphoramidite for 5'-end modification as capping reagent. Tetrahedron, 2024, 150: 133774.https://doi.org/10.1016/j.tet.2023.133774
- Park D, Lee S J, Park J W. Aptamer-based smart targeting and spatial trigger–response drug-delivery systems for anticancer therapy[J]. Biomedicines, 2024, 12(1): 187. https://doi.org/10.3390/biomedicines12010187
- Distributed under Open Access license CC BY 4.0, without modification.
