Bioconjugation Reagents
Bioconjugation Reagents Introduction
Bioconjugation is the formation of complexes by chemically binding functional molecules with biomolecules such as DNA, RNA, proteins, lipids, and sugars under mild conditions.
- Bioconjugation Reagent Overview
- Molecular Properties of Bioconjugation Reagents
- Bioconjugation Reagents Applications
- Quality Control of Bioconjugation Reagents
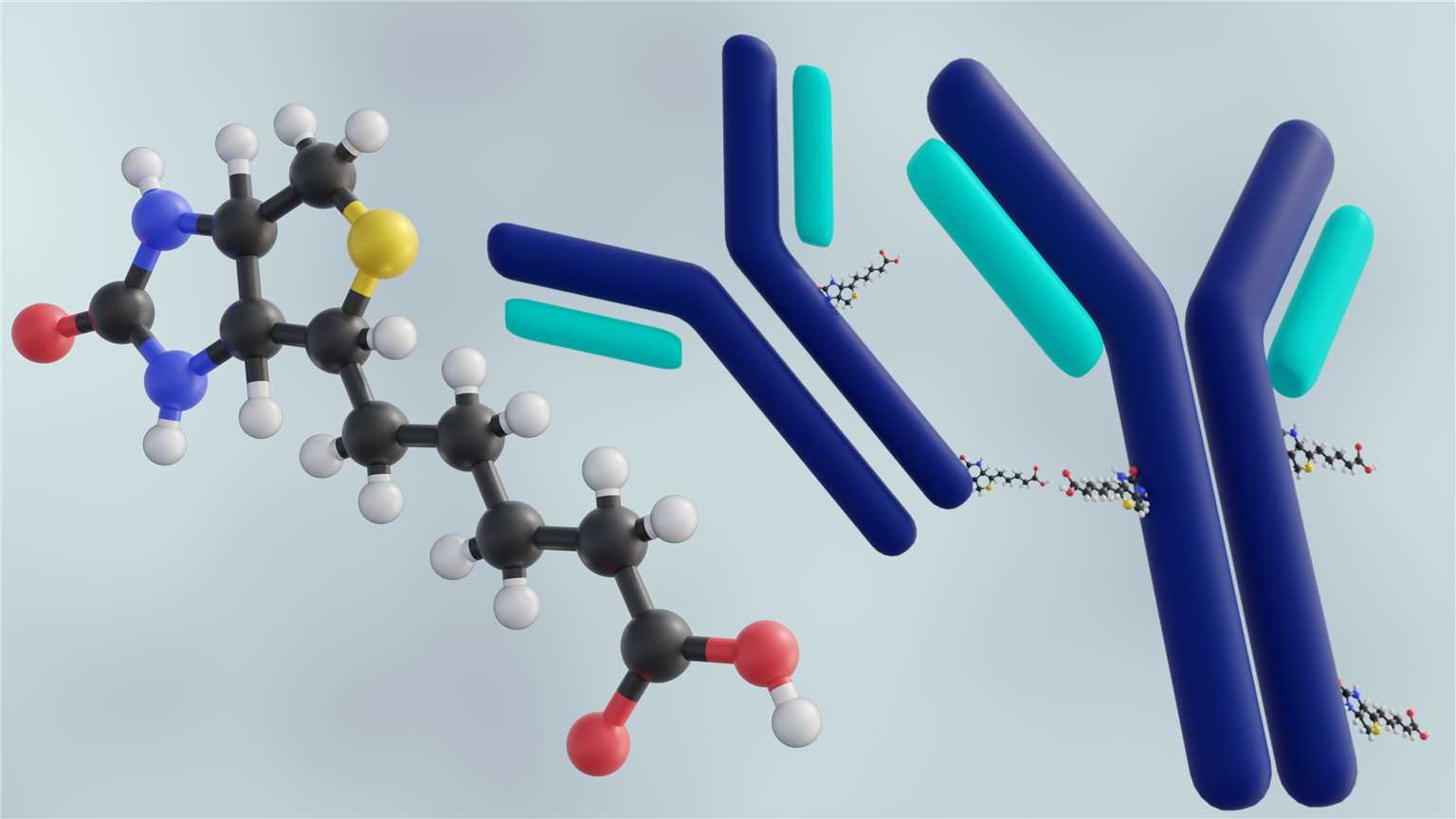
Bioconjugation is a general term to describe the covalent binding of biomolecules (protein, lipid, carbohydrate or nucleic acid) to other small molecules or larger biomolecules. While biomolecule modification has been performed for decades, simple modifications are generally not considered biological coupling (biological coupling is more specifically used to refer to processes where acquisition of biomolecule functionality is involved). For example, bio coupling can be used to attach fluorescent groups, drugs or purification handles to biomolecules, or to link biomolecules to polymer carriers.
- Why are Bioconjugation Reagents Important?
Bioconjugation reagents to enable orthogonal, efficient bioconjugation. Bioconjugation reagents are utilized in the life sciences and medicine for applications in disease diagnostics and targeted therapeutics, as biosensing and fundamental research tools. Targeted drug delivery, diagnostics and research applications all require the modification of biomolecules to improve efficacy, stability, and targeting ability.
Types of Bioconjugation Chemistries and Reagents
Bioconjugation is the preparation of complexes that are formed by covalent bonding of functional molecules to biomolecules such as DNA, RNA, proteins, lipids and sugars under mild conditions. Bioconjugation reagents: biotin, streptavidin, biotinylation reagents, bifunctional cross-linkers, PEG-linkers, auxiliary reagents for click chemistry, etc.
Amine-Reactive Chemistry Reagents
- These chemical methods react with primary amines (lysine residues, N-terminus).
-
Reagents:
- N-Hydroxysuccinimide (NHS) Esters: One of the most commonly used classes of reagents, such as Sulfo-NHS, NHS-PEG. They react with amine groups to form stable amide bonds.
- Isothiocyanates: For example, Fluorescein Isothiocyanate (FITC), often used for fluorescent labeling.
- Succinimides: For example, Maleimide-NHS, which can be used for multi-step conjugations.
- Imidoesters: For example, DMA, DMS, used for reversible crosslinking.
Thiol-Reactive Chemistry Reagents
- These chemical methods react with sulfhydryl groups (cysteine residues).
-
Reagents:
- Maleimides: The most common thiol-reactive reagents, such as SMCC, PEG-Maleimide. They form stable thioether bonds with thiols.
- Haloacetyls: For example, Iodoacetyl, also react with thiols.
- Pyridyl Disulfides: For example, SPDP, which conjugate by forming reducible disulfide bonds.
- Vinyl Sulfones: Form stable covalent bonds with thiols.
Carboxyl-Reactive Chemistry Reagents
- These chemical methods react with carboxyl groups (aspartic acid, glutamic acid, C-terminus).
-
Reagents:
- Carbodiimides (EDC/DIC): Often used in conjunction with NHS to facilitate stable amide bond formation, activating carboxyl groups for conjugation with amines.
Click Chemistry Reagents
- Click chemistry refers to bioorthogonal reactions characterized by high efficiency, specificity, and tolerance to aqueous environments. It has been a rapidly developing conjugation method in recent years.
-
Reagents:
- Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC): Azides and alkynes react in the presence of copper ions.
- Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC): Cyclooctynes and azides react under copper-free conditions, suitable for in vivo conjugation.
- Diels-Alder Reactions: Reactions between tetrazines and trans-cyclooctenes (TCO), known for their speed and high selectivity.
- Oxime/Hydrazone Ligation: Reactions between aldehydes/ketones and aminoxy/hydrazine compounds.
Aldehyde/Ketone-Reactive Chemistry Reagents
- These chemical methods react with aldehyde or ketone groups (e.g., after periodate oxidation of glycans).
- Reagents: Hydrazides, Aminoxy compounds.
Photo-Crosslinking Reagents
- These reagents form covalent bonds upon activation by UV light.
-
Reagents: Aryl azides, Benzophenones.
- Enzyme-Mediated Bioconjugation Reagents
- Utilizes enzymes for highly specific and mild conjugation.
- Examples: Transglutaminase, Tyrosinase.
Key Considerations for Reagent Selection
When selecting reagents for experiments, key considerations include purity, stability, compatibility, and consistency. Researchers must also account for the sensitivity of their experiments to reagent variations and the reproducibility of results using the same reagents.
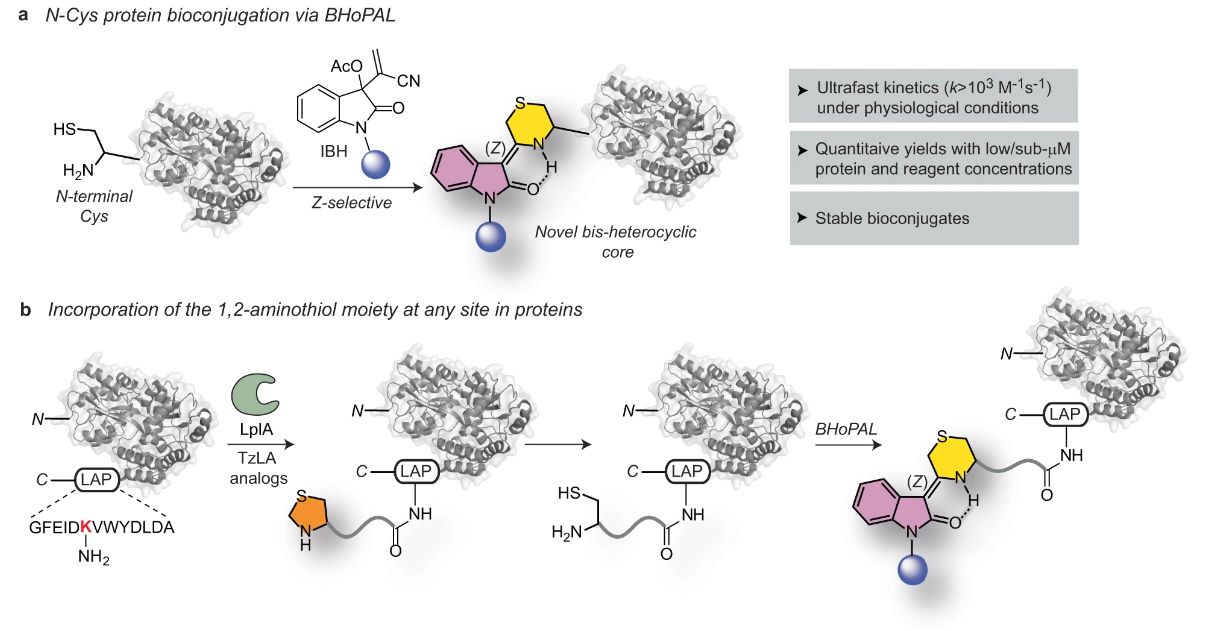 Figure 1 The BHoPAL (Baylis Hillman orchestrated protein aminothiol labelling) strategy for the chemoselective bioconjugation of 1,2-aminothiols in proteins.1
Figure 1 The BHoPAL (Baylis Hillman orchestrated protein aminothiol labelling) strategy for the chemoselective bioconjugation of 1,2-aminothiols in proteins.1
Target Biomolecule Characteristics
- Available Functional Groups: Which amine, thiol, carbohydrate, or other groups on the biomolecule are available for conjugation?
- Stability: What is the stability of the biomolecule under various pH, temperature, and reducing agent conditions?
- Molecular Weight and Structure: How do these properties of the target biomolecule affect conjugation efficiency and product properties?
Reaction Specificity and Efficiency
- Minimizing Non-Specific Reactions: How can reagents be chosen to specifically react with the functional groups of interest?
- Achieving High Conjugation Yield: How can the reaction conditions be optimized to produce the maximum amount of conjugated product?
Linker Properties
- Length and Flexibility: How do these properties of the linker affect steric hindrance and biological activity? Can the linker length be optimized to prevent interference between conjugated molecules?
- Cleavability: Disulfide bonds, acid-labile bonds, or enzyme-cleavable linkers, which are important in certain applications (e.g., drug release).
- Hydrophilicity/Hydrophobicity: How do these properties of the linker affect the solubility and in vivo behavior of the conjugated product?
Reaction Conditions
- pH, Temperature, Solvent Compatibility: How can the reaction conditions be optimized to ensure compatibility with biomolecule stability and conjugation efficiency?
- Presence of Interfering Substances: How do certain buffer components or impurities affect the conjugation reaction?
Biocompatibility
- Toxicity, Immunogenicity: Especially in in vivo applications, what are the toxicity and immunogenicity concerns of the conjugated product?
Uses and Advantages of Bioconjugation Reagents
Bioconjugation reagents can be applied in a wide variety of research applications. These include:
- Western blotting
- ELISA
- ICC
- IHC
- Flow cytometry
- Immuno-PCR
Bioconjugation reagents are useful in various bioprocesses. For example, antibodies labeled directly with enzymes or fluorescent groups can eliminate the secondary antibody incubation step from the immunostaining workflow, accelerate this process, and relatively easily perform a larger number of parallel reads. Antibodies that bind to oligonucleotides provide higher sensitivity in immunoassays and a larger dynamic range than other techniques such as ELISA currently available. Protein conjugates have a similarly broad set of applications as well, whether as immunogens in antibody development (larger carrier proteins can be conjugated to smaller biomolecules to produce a more effective immunogen, for instance), or as diagnostic assay development tools (such as lateral flow assays).
Overview of What Creative Biolabs Can Provide
Creative Biolabs can link two or more biomolecules, such as proteins, nucleic acids, carbohydrates, or small molecules, which is fundamental to a vast array of applications, from diagnostics and therapeutics to advanced materials science and fundamental biological research. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended products
Reference
- Mir M H, Parmar S, Singh C, et al. Location-agnostic site-specific protein bioconjugation via Baylis Hillman adducts. Nature Communications, 2024, 15(1): 859.https://doi.org/10.1038/s41467-024-45124-2. Distributed under Open Access license CC BY 4.0, without modification.
