Custom Nanoparticle-Peptide Conjugation Service
Creative Biolabs provides design, synthesis, and characterization services of peptide gold nanoparticle conjugates for your specific project needs. We can cover all aspects of the project development process from design to final product characterization, backed by our best-in-class instrumentation and many years of professional experience in the nanobiotechnology field. We offer custom peptide synthesis and modification services with several different modification strategies for your peptide. These include adding cysteine residues for thiol coupling to gold nanoparticles, phosphorylated residues for controlling aggregation analysis, and non-natural amino acids for stability and function.
Gold Nanoparticles Labeled Peptides Introduction
Peptide-conjugated gold nanoparticles (AuNPs) represent a cutting-edge platform in nanobiotechnology, combining the unique optical and electronic properties of nanomaterials with the specific biorecognition capabilities of peptides. These hybrid structures exhibit remarkable tunability in their physical and chemical properties depending on their size, shape, and surface chemistry, making them valuable tools for advanced biomedical applications. The importance of peptide-AuNP conjugates stems from their multifunctional capabilities—they simultaneously serve as targeting moieties, therapeutic agents, and diagnostic probes, enabling a true therapeutic approach for disease management, integrating diagnostic and therapeutic functions into a single platform.
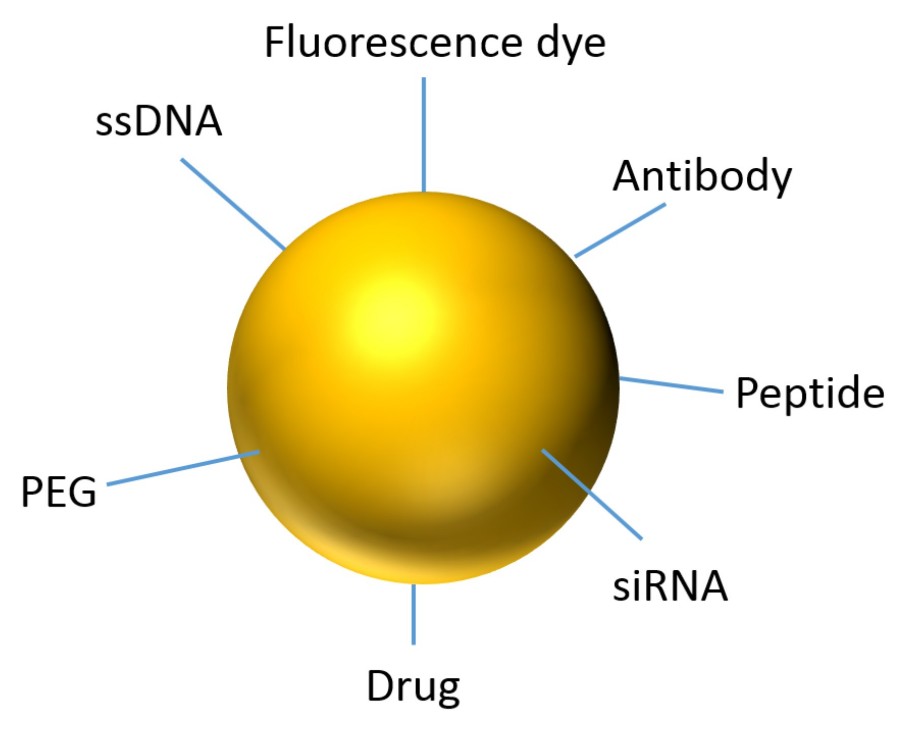 Figure 1. Representation of an Au NP for theranostics.1
Figure 1. Representation of an Au NP for theranostics.1
Conjugation Strategy for Gold Nanoparticles and Peptides
01 Thiol-Au Bonding (Au-S): The Gold Standard for Stability
This is the most common and robust conjugation method, relying on the high affinity of sulfhydryl groups for gold. The inclusion of one or more cysteine (Cys) residues in the peptide sequence provides the necessary sulfhydryl groups to form a strong covalent bond with the gold surface. This method produces conjugates with excellent colloidal stability under complex physiological conditions.
02 Covalent Crosslinking (e.g., EDC/NHS): Suitable for Non-sulfhydryl Peptides
For peptides lacking sulfhydryl groups, mild covalent chemistry is employed. EDC/NHS chemistry is often used to activate carboxyl groups on the peptide, allowing them to react with prefunctionalized amine groups on the AuNP surface to form a stable amide bond. This irreversible binding ensures that the peptide remains anchored even in the presence of competing biomolecules.
03 Non-Covalent Adsorption and Affinity Binding
Non-covalent adsorption and affinity binding This includes simple electrostatic adsorption (e.g., using positively charged peptides and negatively charged citrate-capped AuNPs) and specialized systems such as the biotin-streptavidin affinity system, which exploits one of the strongest non-covalent interactions in nature to achieve highly specific and robust attachment.
Quality Control of Gold Nanoparticles Labeled Peptides
| Parameter | QC Technique | Scientific Justification |
|---|---|---|
| Physical Characterization | ||
| Hydrodynamic Size | Dynamic Light Scattering (DLS) and Zetasizer | Size uniformity is critical for biodistribution and cellular uptake. |
| Core Size & Morphology | Transmission Electron Microscopy (TEM) | Direct visualization confirms the size and shape of the GNP core and the uniformity of the final product. |
| Conjugation Efficiency | UV-Visible Spectroscopy and Thermophoretic Analysis (MTS) | The characteristic SPR peak of the GNP shifts upon conjugation. By measuring the concentration of unbound peptide (e.g., BCA assay or HPLC after separation) and the GNP concentration, the number of peptides per GNP (labeling density) is determined. |
| Chemical & Functional Characterization | ||
| Ligand Confirmation | Fourier-Transform Infrared Spectroscopy (FTIR) or X-ray Photoelectron Spectroscopy (XPS) | Confirms the presence of peptide-specific functional groups on the GNP surface. |
| Bioactivity & Specificity | Surface Plasmon Resonance (SPR) Biosensor or ELISA-like binding assay | Measures the binding kinetics (Kd, kon, koff) of the GNP-peptide conjugate to its target receptor. This is the most crucial step to ensure the conjugation process did not compromise the peptide's biological function. |
| Stability | Accelerated Stability Testing (e.g., at 4∘C and room temperature) in biological buffers (PBS, serum) | Measures changes in DLS size and SPR over time to confirm resistance to aggregation and ligand exchange in vitro. |
Applications of Gold Nanoparticles Labeled Peptides
Targeted Drug Delivery and Cancer Therapy
Peptide-AuNP conjugates have revolutionized targeted cancer therapy, enabling precise delivery of therapeutics to malignant cells while minimizing off-target effects. These constructs exploit the enhanced permeability and retention (EPR) effect of the tumor vasculature for passive targeting, while the peptide ligand provides active targeting to specific cell surface receptors overexpressed in cancer cells.
Diagnostic and Bioimaging Applications
The exceptional optical properties of gold nanoparticles, combined with the precise targeting capabilities of peptides, enable advanced diagnostic and bioimaging applications. Peptide-AuNP conjugates serve as potent contrast agents for various imaging modalities, including electron microscopy, optical and confocal microscopy, and surface-enhanced Raman spectroscopy (SERS). The small size (as low as 1.4 nm) and uniform distribution of these conjugates allow for high-resolution imaging, while their low background signal enhances detection sensitivity.
Future of Gold Nanoparticles Labeled Peptides
Therapeutics: Combining diagnostic (imaging/sensing) and therapeutic (drug delivery/photothermal) capabilities into a single GNP peptide structure is the main direction. A single reagent can non-invasively detect tumors, deliver drug payloads, and activate local thermal ablation through light (such as photothermal therapy, PTT).
Intelligent system: Develop "intelligent" conjugates that respond to specific disease microenvironments (such as low pH in tumors, high enzyme concentrations in inflammation) by structural changes or releasing their cargo only at the target site. This requires highly complex and cleavable peptide linker design.
Expanding scale and GMP production: Transitioning from laboratory scale synthesis to Good Manufacturing Practice (GMP) production is crucial for clinical trials. This requires standardized, reproducible, and cost-effective conjugate schemes.
Core Services at Creative Biolabs
Creative Biolabs is your expert partner for state-of-the-art bioconjugation, specializing in the design, synthesis, and detailed characterization of gold nanoparticle-labeled peptides.
| Labeling Services | Specification |
|---|---|
| Gold Nanoparticle Labeled Signal Peptides | Creative Biolabs specializes in integrating nanotechnology with signaling molecules. By efficiently conjugating gold nanoparticles (GNPs) to signaling peptides, we provide cutting-edge solutions for ultrasensitive visualization and detection of these critical biological events, significantly advancing cell signaling research. |
| Gold Nanoparticle Labeled Neuropeptides | Neuropeptides are short peptides that regulate and transmit information within the nervous system. Creative Biolabs utilizes advanced bioconjugation chemistry to ensure stable anchoring of neuropeptides to the surface of functionalized GNPs through highly stable covalent or non-covalent bonding strategies. |
| Gold Nanoparticle Labeled Antimicrobial Peptide | Our technicians employ advanced surface modification and conjugation techniques to precisely attach AMPs to the GNP surface, enhancing their stability and local concentration—a phenomenon known as the multivalency effect. |
| Gold Nanoparticle Labeled Cell-penetrating Peptide | Cell-penetrating peptides (CPPs) are a unique class of short peptides that can effectively cross cell membranes or tissue barriers. Based on customer needs, Creative Biolabs precisely surface-functionalizes gold nanoparticles to optimize CPP binding efficiency and stability. |
Our Workflow
Our systematic workflow ensures the highest quality and scientific rigor for every gold nanoparticle-labeled peptide project.
![]()
Request for Evaluation
Our Ph.D.-level scientists will conduct an in-depth review of your target application, peptide sequence, and desired gold nanoparticle (GNP) specifications (size, concentration, linker type).
![]()
Experimental Design
We will develop a detailed conjugation strategy, including a proposed bulk gold nanoparticle synthesis, peptide modification, and quality control plan, to meet your specific functional requirements.
![]()
Quote Approval
The project will officially begin upon client approval of the scientific plan and associated costs.
![]()
GNP-labeled Peptide Synthesis
We will synthesize the core gold nanoparticles (GNPs), followed by surface modification and controlled bioconjugation of the peptide under optimized conditions.
![]()
Result Review
We will conduct comprehensive quality control (DLS, UV-Vis, and HPLC analysis of unbound peptide) and prepare a detailed report for your review.
![]()
Deliverables
Highly pure, characterized gold nanoparticle-labeled peptides, along with a complete quality control report, will be delivered to your laboratory.
Frequently Asked Questions
Q: What are the main advantages of using gold nanoparticles to label peptides compared to fluorescent dyes?
A: The main advantages are signal amplification and stability. Gold nanoparticles have excellent light scattering and surface plasmon resonance (SPR) enhancement properties, resulting in higher intensity and greater resistance to photobleaching than traditional organic fluorescent dyes. This significantly improves the sensitivity of diagnostic assays and allows for applications such as colorimetric detection and photoacoustic imaging. Furthermore, the high surface area of gold nanoparticles (GNPs) enables the binding of large numbers of peptides (multivalent states), significantly enhancing binding affinity to the target.
Q: How does the size of the gold nanoparticles affect the final labeled peptide product?
A: Size is a key factor in determining the properties of the conjugate.
Smaller gold nanoparticles (∼5-20 nm) are well-suited for targeted drug delivery and cellular uptake because they are small enough to penetrate tissues and be efficiently absorbed through endocytosis. They also exhibit the most pronounced SPR color change upon aggregation, making them useful in diagnostic assays. Larger GNPs (approximately 40-100 nm): Due to their larger mass and higher X-ray absorption, they are more suitable for certain bioimaging modalities (such as CT angiography), but they may face challenges with tissue penetration and circulation. For larger particles, the SPR peak shifts toward longer wavelengths (redder).
Q: What are the biggest challenges in conjugating peptides to GNPs for in vivo applications?
A: The primary challenges are colloidal stability and avoiding nonspecific binding in vivo. Biological fluids (such as plasma) contain high concentrations of salts and proteins, which can destabilize the GNP surface, leading to aggregation (loss of function) or displacement of the peptide ligand by endogenous proteins (protein corona formation). This challenge can be alleviated through surface passivation, typically through coadsorption or grafting of polyethylene glycol (PEG) chains, which provide a steric barrier and impart hydrophilicity.
Conclusion
Creative Biolabs specializes in developing targeted therapeutic conjugates for drug delivery, diagnostic probes for biosensing, and multifunctional theranostic platforms that combine therapeutic and monitoring capabilities. Our team is particularly adept at developing stable, high-affinity conjugates for challenging applications such as brain targeting, tumor-specific drug delivery, and intracellular imaging. Please contact us to discuss your demands or to request a proposal.
Reference
- Siddique S, Chow J C L. Gold nanoparticles for drug delivery and cancer therapy. Applied Sciences, 2020, 10(11): 3824. https://doi.org/10.3390/app10113824 (Distributed under Open Access license CC BY 4.0, without modification.)
