Custom Click Chemistry based Conjugation Service
Conventional conjugation methods often lead to heterogeneous products. In contrast, site-specific conjugation offers a targeted approach by coupling molecules at defined sites, thereby increasing consistency and efficacy. With years of experience in bioconjugation, Creative Biolabs offers click chemistry-based conjugation services to improve research efficiency for our customers.
Introduction of Click Chemistry
Click chemistry (also called "ligand chemistry") is a chemical reaction based on the formation of carbon-heteroatom bonds (C-X-C) to efficiently and reliably synthesize a diverse array of molecules through the assembly of small units. This concept was first introduced by chemist K. Barry Sharpless in 2001, which quickly attracted widespread attention across various fields, including molecular biology, materials science, and drug discovery. The high efficiency and multifunctionality of click chemistry make it particularly valuable in applications related to bioconjugation.
Key Features of Click Chemistry
- High selectivity: These reactions are typically highly chemoselective and can specifically link target molecules under complex reaction conditions, thereby minimizing the formation of by-products.
- Mild reaction conditions: Many click reactions can be conducted under relatively gentle conditions, which reduces the demands on both the environment and the experimental setup.
- High efficiency: Click chemistry reactions are characterized by their high reaction rates and yields, which allow for the rapid completion of synthetic processes, thus greatly reducing experimental time.
- Excellent functional group tolerance: Many classic click reactions, such as the azide-alkyne cycloaddition, can tolerate a wide range of functional groups, including amines, carboxylic acids, esters, alcohols, and ethers. By exploiting this characteristic, chemists can preserve the reactivity of different functional groups during reactions, increasing the variety of compounds that can be created.
Conjugation Methods Based on Click Chemistry
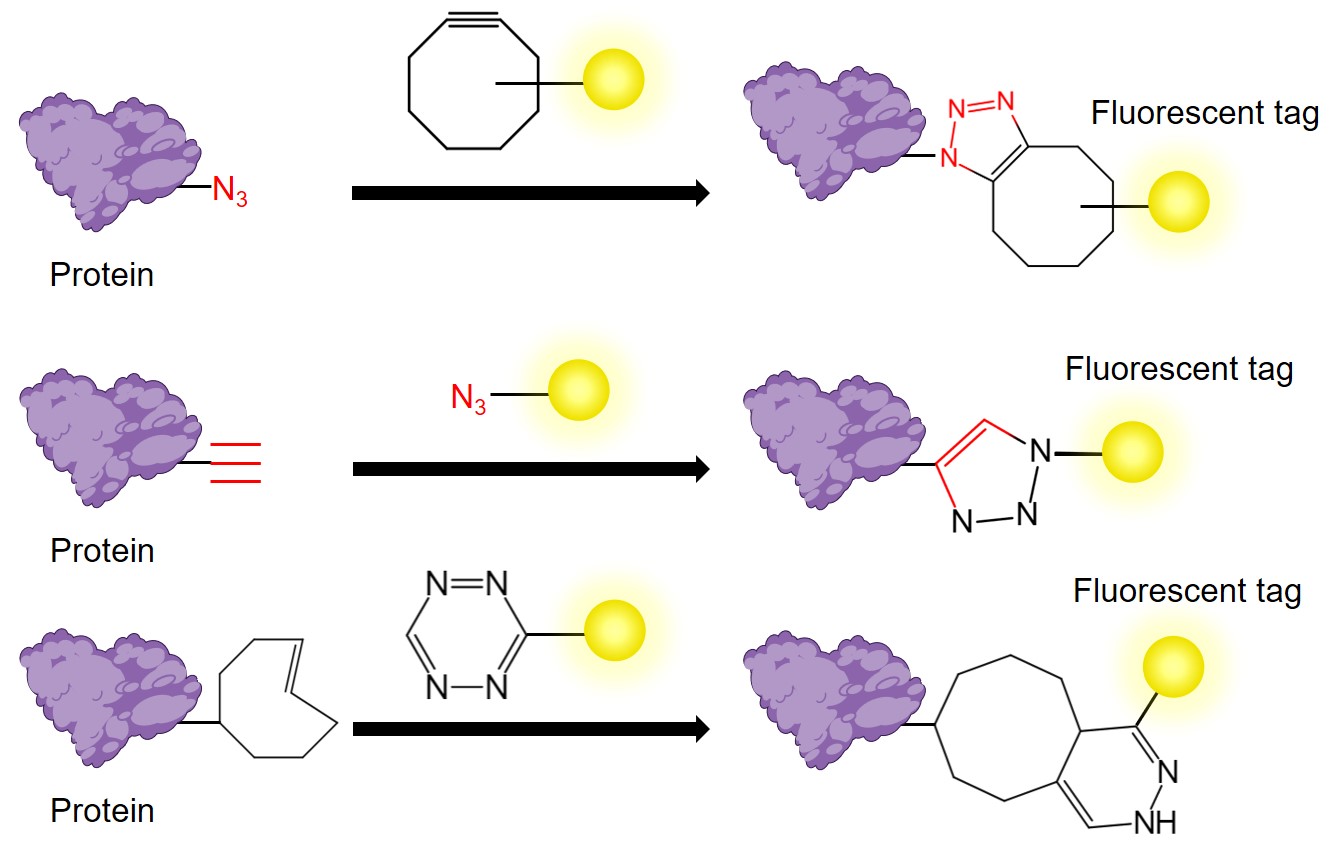 Fig.1 Three common types of click chemistry.
Fig.1 Three common types of click chemistry.
Copper(I)-Catalyzed Alkyne-Azide Cycloaddition (CuAAC)
CuAAC is a hallmark reaction in click chemistry. It utilizes Cu(I) as a catalyst to achieve a highly selective conjugation of azides and alkynes, resulting in the formation of 1,4-disubstituted 1,2,3-triazoles. This reaction can occur over a broad temperature range and is effective across a wide pH spectrum from 4 to 12. Furthermore, CuAAC exhibits insensitivity to water and demonstrates tolerance to many functional groups, making it a valuable tool in biolabeling and drug synthesis.
Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC)
SPAAC is a copper-free cycloaddition reaction involving high-strain cyclic alkynes and azides. This reaction leverages the released energy from the ring strain during the transformation into stable 1,2,3-triazoles. By eliminating the need for metal catalysts, SPAAC circumvents the potential toxicity of copper, making it highly suitable for biological systems, particularly in applications involving in vivo labeling.
Inverse Electron Demand Diels–Alder Reaction (iEDDA)
iEDDA refers to the [4+2] cycloaddition reaction occurring between electron-deficient diene and electron-rich dienophile, which can proceed under mild conditions. Compared to the traditional Diels-Alder reaction, iEDDA is considered a bioorthogonal reaction and exhibits significantly faster reaction rates. Therefore, it holds greater potential for applications in bioconjugation.
Thiol-Maleimide Reaction
The thiol-maleimide reaction is a nucleophilic addition reaction of a thiol with a maleimide to form a thiosuccinimide product. It is characterized by its rapid reaction rate and mild conditions, allowing it to be carried out in aqueous solvents or even in the absence of solvents. Moreover, the readily available reagents make the thiol-maleimide reaction one of the most active click reactions for synthesizing antibody-drug conjugates (ADCs).
Creative Biolabs offers a comprehensive range of conjugation services based on click chemistry strategies, catering to various fields including proteins, nucleic acids, small molecule drugs, and nanoparticles. If your research is looking for a suitable conjugation service provider, please contact us now! Our team of professionals will collaborate closely with you to develop custom solutions to match your specific research requirements.
Reference
- Hammond, Suzan M., et al. "Delivery of oligonucleotide‐based therapeutics: challenges and opportunities." EMBO molecular medicine 13.4 (2021): e13243. Distributed under Open Access license CC BY 4.0, without modification.
Related products
