Custom Antibody-siRNA Conjugation Service
What is Antibody siRNA Conjugate?
Antibody drug conjugates can target therapeutic drugs to the desired lesion area and have achieved considerable market value. However, the relatively low drug load of ADC systems often results in the inability to deliver sufficient chemical drugs to the desired area with safe antibody doses, thereby limiting the efficacy of ADC systems. With the approval of five small interfering RNA (siRNA) drugs patisiran, givosiran, lumasiran, inclisiran, and vutrisiran, the development of siRNA has once again attracted widespread attention from researchers. siRNA can achieve good therapeutic effects at doses of nmol or lower and has good specificity. However, due to its poor cell membrane permeability and undesirable toxicity, safely and effectively delivering siRNA to target cells is the main obstacle to advancing its clinical application. Based on the concept of ADC delivery system, antibody siRNA conjugates (ARC) have emerged, which are expected to overcome many obstacles and achieve low toxicity, long blood circulation time, and high targeting ability through fast and simple preparation procedures.
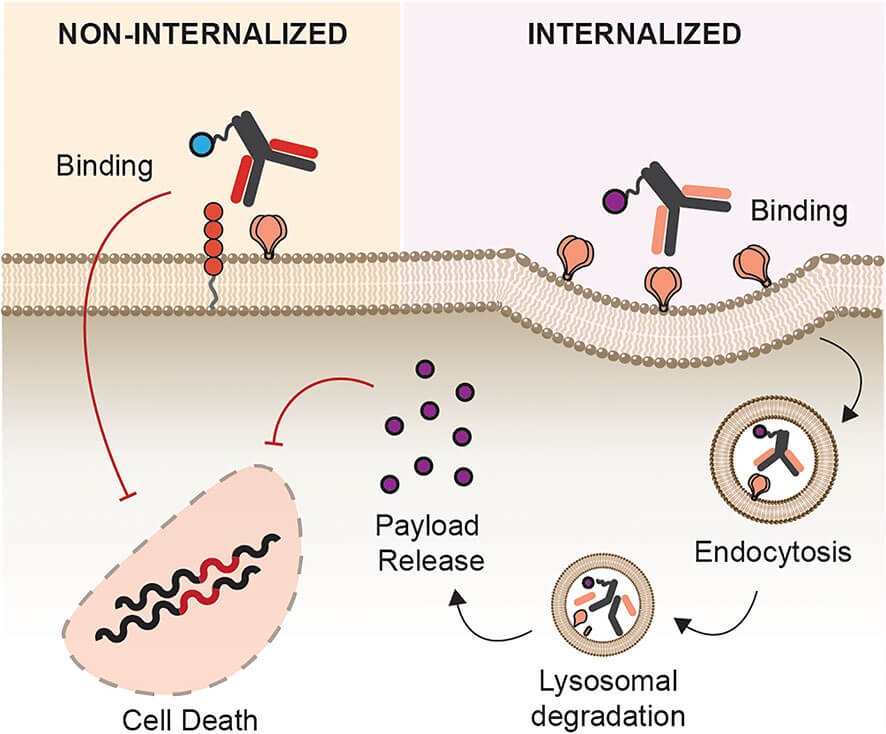 Figure 1 Site of Action. Antibody conjugates can act either intracellularly or intercellularly upon antigen binding.1
Figure 1 Site of Action. Antibody conjugates can act either intracellularly or intercellularly upon antigen binding.1
What Are the Difficulties to Develop a Therpeutic siRNA Drug?
The development of therapeutic small interfering RNA (siRNA) drugs faces significant challenges that limit their clinical translation, particularly for diseases outside the liver. siRNA works through RNA interference (RNAi), which is a natural cellular process that mediates sequence specific gene silencing by degrading complementary mRNA molecules. Although this mechanism provides great potential for treating diseases by targeting previously "untreatable" pathways, the development of siRNA therapy has encountered multiple obstacles:
- Poor In Vivo Stability
- Inefficient Cellular Uptake
- Off-Target Effects
- Lack of Tissue and Cell-Specificity
Antibody siRNA Conjugate: Emerging New siRNA Drug Formulation
ARCs combine the targeting accuracy of monoclonal antibodies with the gene silencing activity of siRNA, creating a "guided RNAi" platform that addresses the limitations of free siRNA. Like ADC, ARCs consist of three parts: ligands targeting mAbs, siRNA payloads, and biocompatible adapters, but there are key design considerations for the unique characteristics of siRNA.
Conjugation Strategies: Site-Specific vs. Random
The selection of coupling sites directly affects the stability, target binding affinity, and siRNA activity of ARC. Random binding (e.g. through lysine residues on the Fc region of antibodies) is simple, but can result in heterogeneous mixtures with variable payloads (2-8 siRNA per antibody), leading to inconsistent pharmacokinetics.
Linker Design for siRNA Delivery
ARC junctions must balance stability in circulation and trigger release in target cells. The acid unstable linker is stable at physiological pH (7.4), but degrades in the acidic endosome, releasing siRNA into the cytoplasm. Enzyme cleaved linkers (such as cathepsin B sensitive peptides) utilize overexpression of proteases in diseased cells (such as cancer) for site-specific payload release.
Overcoming siRNA Limitation
ARCs alleviate the core challenge of siRNA therapy:
- Stability: The antibody scaffold protects siRNA from nucleases and prolongs its circulating half-life.
- Cell uptake: Compared to free siRNA, antibody mediated endocytosis increases siRNA internalization by 10-100 times.
- Specificity: Targeting antigens limited to diseased cells can reduce off target accumulation and immune activation.
- Tissue penetration: Compared to full-length monoclonal antibodies, smaller antibody forms such as Fab fragments and single chain variable fragments [scFvs] can enhance the penetration of solid tumors and dense tissues (such as the brain).
Applications of Antibody siRNA Conjugate
The application potential of ARC spans multiple therapeutic fields, providing a new therapeutic paradigm for diseases that have been proven resistant to traditional methods.
Oncology Applications
In cancer treatment, ARC is able to accurately deliver siRNA payloads to tumor cells while preserving healthy tissue, addressing the significant challenge of tumor specific delivery that hinders traditional siRNA therapy.
Genetic Disorder
ARC has shown particular promise in the treatment of hereditary muscle diseases, such as type 1 myotonic dystrophy (DM1), Duchenne muscular dystrophy (DMD), and facial, shoulder, and humeral muscular dystrophy (FSHD).
Central Nervous System Diseases
Due to the impermeable blood-brain barrier, the treatment of neurological diseases is a particularly challenging application area. However, ARC targeting transferrin receptors or other receptors involved in endocytosis has shown the potential to deliver siRNA payloads to the brain.
Overview of What Creative Biolabs Can Provide
At Creative Biolabs, we leverage our extensive expertise in antibody engineering and conjugation chemistry to provide comprehensive services for the development of antibody siRNA conjugates. Our end-to-end solution provides researchers with support from initial concepts to preclinical validation, accelerating the transition of ARC therapy from the laboratory to the bedside.
- siRNA design, synthesis, purification, characterization
- Antibody production/custom synthesis
- Conjugate the antibody and siRNA
- Characterization
Why Choose Our Services?
 Proprietary Conjugation Platforms
Proprietary Conjugation Platforms
Our technology can achieve site-specific coupling with>90% efficiency, producing uniform ARCs with minimal loss of antibody or siRNA activity. Unlike general service providers, we have optimized the coupling conditions of each antibody siRNA pair to ensure large-scale reproducibility.
 Multidisciplinary Expertise
Multidisciplinary Expertise
Our team includes doctoral level scientists specializing in antibody engineering, RNA biology, bio coupling chemistry, and preclinical pharmacology, all under one roof. This integrated approach eliminates the bottleneck between discovery, conjugation, and characterization.
 Fast Turnover and Scalability
Fast Turnover and Scalability
We offer a fast turn-over for screening candidate lead compounds (siRNA design+antibody binding assay 2-4 weeks) and scalable production (from milligram to gram quantities) for preclinical and early clinical studies. Our compliant facilities allow for smooth transition to clinical production.
 Customization and Flexibility
Customization and Flexibility
We customize our services to the specific needs of your program. Whether you need a turnkey, end-to-end development, or independent integration/characterization steps, we tailor the service package and timelines to your specific project needs. Our scientists interact with your team to modify protocols and prioritize milestones.
Customer Review
Reference
- Umotoy J C, De Taeye S W. Antibody conjugates for targeted therapy against HIV-1 as an emerging tool for HIV-1 cure. Frontiers in immunology, 2021, 12: 708806. https://doi.org/10.3389/fimmu.2021.708806 Distributed under Open Access license CC BY 4.0, without modification.

