Immunopathology Complement Activation Complement Molecular Mechanisms Related Products Hot Services Q&A Resources
Are you currently facing the daunting challenges of high mortality rates, limited treatment options, and complex diagnostic hurdles in rare neurological infections? Creative Biolabs’ Complement Pathway Modulators help you overcome therapeutic hurdles and develop life-saving treatments for devastating diseases like PAM through innovative complement inhibition strategies and targeted therapeutic molecule design.
Immunopathology Features
Primary Amebic Meningoencephalitis (PAM)
Primary amebic meningoencephalitis (PAM) is a rapidly progressive and potentially fatal infection caused by Naegleria fowleri. The free-living amoebae is world widely found in natural warm bodies of fresh water, such as lakes, rivers, and hot springs, and prefers temperatures between 25°C~40°C. It can also occur in warm water discharge from industrial plants, under-chlorinated swimming pools, and soil. Although it rarely causes disease, the inflammation and destruction of the central nervous system (CNS) and the linings of the brain is usually fatal. The infection of Naegleria fowleri is usually initiated from the nose, through activities such as jumping, diving or falling into the contaminative water, then travels up to the brain. The disease cannot be contracted by drinking water or through contact transmission.
Symptoms of Primary Amebic Meningoencephalitis
In the early stage of PAM infection, signs and symptoms may be very similar to those of bacterial meningitis.
-
Initial symptoms of PAM
-
Severe and persistent headache
-
High fever
-
Nausea and vomiting
-
Sleepiness
-
Sore throat
-
Later symptoms of PAM
-
Stiff neck
-
Confusion
-
Lack of attention to people and surroundings
-
Loss of balance
-
Seizures
-
Hallucinations
After the start of symptoms, the disease progresses rapidly and usually causes death within about 5 days.
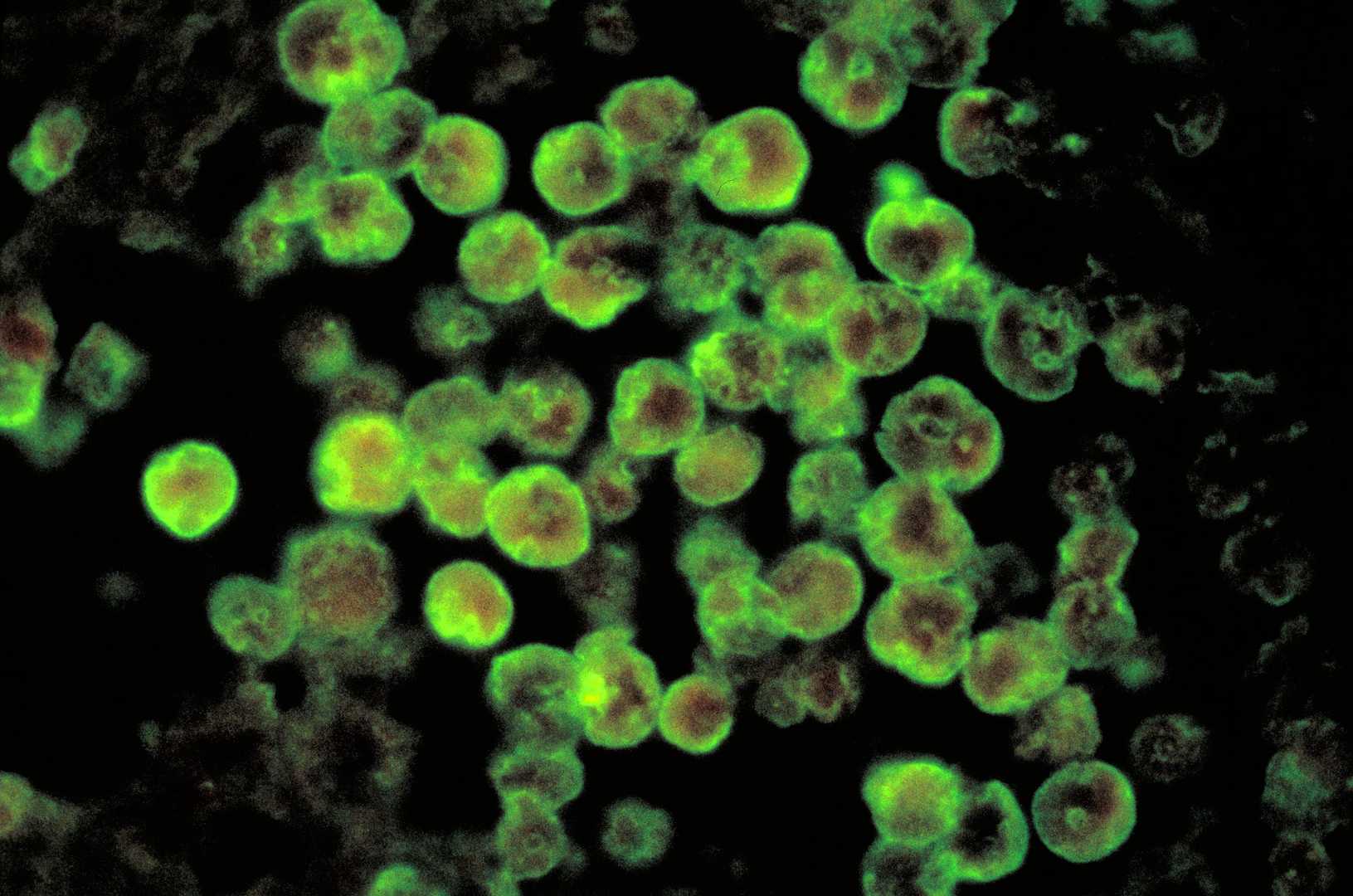
Fig. 1 Histopathological features of amebic meningoencephalitis visualized by direct fluorescent antibody staining.1
Pathophysiology of Primary Amebic Meningoencephalitis
An amplified host immune response is thought to contribute to the pathophysiology of PAM, which can stride over the blood-brain barrier, causing an inflammatory reaction and subsequent neuronal and parenchymal damage. In PAM patients, the release of acute inflammatory cytokine, including acid hydrolases, phospholipases, neuraminidases, and phospholipolytic enzymes also play roles in the pathogenicity of PAM. In response to Naegleria fowleri infection, the organism triggers a variety of immune response to clear the pathogen.
-
Complement
Using a mouse model of PAM, researchers found that complement C5 deficient individual is more susceptible to PAM. Strong evidence revealed that the nonpathogenic or weakly pathogenic species of Naegleria fowleri are complement-sensitive and can be lysed by the MAC of complement. Unfortunately, highly pathogenic Naegleria amebae can resist complement damage via two ways: (1) expression of complement-regulatory proteins; (2) shedding of the MAC (C5b-C9) on vesicles. The identified ‘CD59-like’ molecule on the surface membrane of Naegleria fowleri can interact with protein kinases, resulting in vesiculation and removal of the lytic complex, C5b-C9, from the ameba membrane surface.
-
Neutrophils
During Naegleria fowleri infection, the activated neutrophils markedly increase and aggregate at focal sites harboring amebae to play a role in the destruction of amebae. TNF-a can augment the neutrophils mediated immune responses.
-
Macrophages
Accumulating evidence showed that macrophages act in host defense against Naegleria fowleri. They serve as efficient cytotoxic cells against pathogens by producing reactive oxygen intermediates and eliciting nitric oxide (NO) and nonoxidative mediators including TNF-a and IL-1 to lyse amebae.
Related Complement Activation Pathway
Table 1 Complement activation pathways.
|
Complement Activation Pathways
|
PAM Pathogenesis
|
|
Classical Pathway
|
The classical pathway is generally initiated by antibody-antigen complexes or C-reactive protein binding to pathogen surfaces; however, in the initial phase of PAM, Naegleria fowleri can activate the pathway in vitro without relying heavily on antibodies. Both pathogenic and non-pathogenic Naegleria species can activate the complement cascade, producing C3 and C5 cleavage products, yet highly pathogenic Naegleria fowleri strains exhibit significant resistance to complement-mediated lysis, underscoring a critical virulence factor.
|
|
Alternative Pathway
|
Highly pathogenic Naegleria fowleri directly activates the Alternative Pathway due to the lack of complement regulatory proteins on its surface. Naegleria fowleri demonstrates significant resistance to complement-mediated lysis through mechanisms such as expressing complement-regulatory protein mimics like a "CD59-like" molecule, shedding membrane vesicles containing the MAC, and possessing glycoproteins on its surface.
|
|
Lectin Pathway
|
While the lectin pathway's role in Naegleria fowleri pathogenesis is less studied, the presence of surface glycoconjugates might activate this pathway.
|
Molecular Mechanisms of Complement-Mediated
Table 2 Molecular mechanisms of complement-mediated.
|
Key Complement Components
|
Functions
|
|
CD59
|
Pathogenic Naegleria fowleri expresses a "CD59-like" membrane protein that cross-reacts with antibodies against human CD59 and can immunoprecipitate with human complement component C9, suggesting that it disrupts MAC formation by mimicking host complement regulatory proteins, thus shielding itself from lysis—a feature absent in complement-sensitive, non-pathogenic Naegleria fowleri species.
|
|
C3b and components of the MAC
|
The amoeba Naegleria fowleri employs a defense strategy by peeling off sections of its membrane embedded with complement proteins (C3b and MAC), which serves as a diversionary tactic to intercept the MAC and avert cellular lysis, while simultaneously attracting further complement fragments, thereby safeguarding its core structure.
|
Creative Biolabs has established advanced Complement Therapeutics Platform including antibody engineering platform, protease inhibitor platform, and drug discovery platform, and is equipped to offer a full range of biotherapeutics development services regarding drug discovery and validation for PAM. Please feel free to contact us for detailed information.
Related Hot Products
Our comprehensive complement platform offers a wide array of diverse and cost-effective complement-associated products, and we encourage you to contact us.
Table 3 Featured products.
Related Hot Services
Table 4 Complement test services for SS-related complement studies.
Resources
Reference
-
By Photo Credit:Content Providers(s): CDC/ Dr. Govinda S. Visvesvara - This media comes from the Centers for Disease Control and Prevention's Public Health Image Library (PHIL), with identification number #408., Public Domain, https://commons.wikimedia.org/w/index.php?curid=930518
For Research Use Only.
Related Sections: