Introduction What We Can Offer? Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Are you currently facing challenges in understanding the complex interplay of the complement system in cardiovascular diseases, or struggling to develop targeted therapies? Creative Biolabs helps you accelerate drug discovery and obtain high-quality research insights by leveraging advanced recombinant DNA technology and high-throughput screening platforms, specifically focusing on the complement system's role in cardiovascular health and disease.
Contact our team to get an inquiry now!
Introduction
The complement network, a fundamental element of innate immunity, protects against pathogens while eliminating immune aggregates and programmed cell death remnants. A cascade of plasma proteins and cell surface receptors, it activates via classical, lectin, and alternative pathways, converging to C3 and C5 convertases, generating inflammatory mediators (C3a, C5a) and the membrane attack complex (MAC, C5b-9). Though vital for immune surveillance, its dysregulation or excessive activation significantly contributes to various cardiovascular diseases (CVDs).
Click here to learn more information about specific CVDs
Complement System in Cardiovascular Disease
Recent literature highlights the complement's "double-edged sword" role in cardiovascular health. In myocardial ischemia/reperfusion (I/R) injury, complement activation worsens tissue damage, causing cardiomyocyte death and inflammation. In atherosclerosis, chronic complement activation promotes plaque formation and instability. Anaphylatoxins C3a, C5a, and MAC drive inflammatory responses, recruit immune cells, and damage endothelial cells, advancing conditions like heart failure, hypertension, and aortic aneurysm. Understanding these interactions and activation mechanisms in CVDs is critical for effective therapeutic strategies.
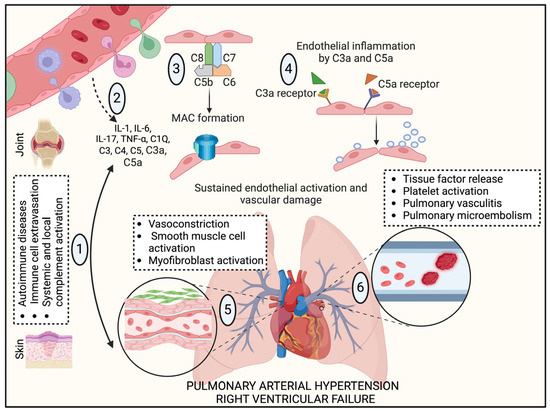 Fig.1 Autoimmune disorders and complement cascade initiation in PAH pathological development.1
Fig.1 Autoimmune disorders and complement cascade initiation in PAH pathological development.1
Cardiovascular disease (CVD), a major cardiometabolic disorder, involves critical narrowing or occlusion of blood vessels. The complement system is implicated in pivotal CVD processes, including endothelial impairment, atherosclerosis, and altered coagulation/fibrinolysis.
Complement's Role in Endothelial Dysfunction
Endothelial dysfunction reduces vasodilation and promotes inflammatory cell adherence, initiating atherosclerosis. Key complement components (C3a, C5a, C5b-9) trigger adhesion molecule and pro-inflammatory cytokine expression in endothelial cells.
Atherosclerosis Development and Complement's Involvement
Complement activation occurs from nascent fatty streaks to advanced atherosclerotic plaques. Components are found within atheromas, often co-localizing with activators like CRP, oxidized LDL, and macrophages (e.g., C1q, C1r, C1s, C4, C7, C8 in human plaques). Mannose-binding lectin (MBL), a lectin pathway factor, is significant in coronary artery disease and myocardial infarction pathogenesis, impacting plaque formation, destabilization, and thrombotic events.
Complement's Influence on Coagulation and Fibrinolysis in CVD
Complement protein C3 demonstrates biochemical association with fibrinogen, thrombocyte levels, and PAI-1. Plasma C3 concentration correlates with its integration into fibrin clots, a recognized structural element of blood clots.
What We Can Offer?
Creative Biolabs provides a comprehensive suite of products and services to empower your research and therapeutic development targeting the complement system in cardiovascular disease:
-
Recombinant Complement Proteins: High-purity, functionally active recombinant proteins of various complement components (e.g., C1q, C3, C5, Factor B, Factor D, Properdin, MBL) for mechanistic studies and assay development.
-
Anti-Complement Antibodies: A diverse catalog of highly specific monoclonal and polyclonal antibodies targeting key complement proteins and their activated fragments (e.g., anti-C3a, anti-C5a, anti-C5, anti-C1q) for research, diagnostic, and therapeutic applications.
-
Complement Inhibitors: A range of small molecule and biologic inhibitors targeting different points in the complement cascade (e.g., C1-inhibitors, C3 inhibitors, C5 inhibitors, anti-C5aR antibodies) for in vitro and in vivo studies.
-
Complement Activity Assays: Comprehensive functional assays to measure classical, alternative, and lectin pathway activation, as well as specific component activation (e.g., C3a, C5a, sC5b-9 ELISA kits).
Why Choose Us?
Creative Biolabs navigates pioneering pathways in complement system investigation and cardiovascular treatment innovation. Our commitment to scientific excellence and innovation ensures that our clients receive unparalleled support and solutions.
-
Deep Expertise: With decades of experience in immunology and cardiovascular biology, our team possesses profound knowledge of the complement system and its intricate roles in health and disease.
-
Comprehensive Platforms: We utilize state-of-the-art platforms for complement protein production, antibody development, and functional assay design, enabling precise and high-throughput analysis.
-
High-Quality Reagents: We provide a wide array of rigorously validated recombinant complement proteins, specific antibodies, and inhibitors, ensuring reproducibility and reliability in your experiments.
-
Customized Solutions: Recognizing the unique nature of each project, we offer tailored services, from target identification and validation to preclinical model development and drug screening.
-
Proven Track Record: Our methodologies and products have contributed to significant advancements in complement research, as evidenced by Published Data demonstrating successful outcomes in various cardiovascular disease models.
-
Dedicated Support: Our scientific team offers expert consultation and technical support throughout your project, ensuring seamless execution and optimal results.
Secure the Creative Biolabs Edge – Solicit Specifications Now
FAQs
Q: How can modulating the complement system impact cardiovascular disease progression?
A: Targeting specific components or pathways within the complement system can significantly influence the inflammatory and damaging processes that drive cardiovascular diseases. By inhibiting excessive complement activation, it's possible to reduce inflammation, prevent cell and tissue damage, and potentially slow or even reverse disease progression in conditions like heart attack recovery or atherosclerosis. The precise impact depends on the specific disease and the component being modulated.
Q: Are there different strategies for inhibiting the complement system, and which is most effective for cardiovascular applications?
A: Yes, there are various strategies, including blocking upstream activators, inhibiting convertases, or neutralizing downstream effectors like C3a, C5a, or the MAC. The most effective strategy often depends on the specific cardiovascular disease and the precise mechanism of complement involvement. For example, in acute conditions like ischemia-reperfusion injury, targeting early components might be beneficial, while in chronic diseases like atherosclerosis, a more sustained or specific inhibition might be preferred. Researchers often explore different approaches to find the optimal therapeutic window.
Q: What are the potential challenges or side effects associated with complement inhibition in cardiovascular settings?
A: While complement inhibition holds great promise, a key challenge is maintaining the system's essential protective functions while mitigating its detrimental effects. Over-inhibition could potentially increase susceptibility to certain infections, as the complement system is vital for host defense. Therefore, developing highly specific inhibitors that selectively target pathogenic complement activation, or strategies that achieve transient inhibition, are crucial to minimize off-target effects and ensure patient safety.
Q: How does complement inhibition compare to existing treatments for cardiovascular diseases?
A: Complement inhibition represents a novel therapeutic approach that directly addresses a fundamental inflammatory mechanism often overlooked by traditional treatments. While existing treatments might manage symptoms or risk factors, complement-targeted therapies aim to intervene at a deeper pathological level. They can potentially be used as monotherapies or in combination with current standards of care to provide enhanced protection and improve patient outcomes, especially in cases where inflammation plays a dominant role.
Q: What kind of data or evidence is typically needed to demonstrate the efficacy of a complement-targeted therapeutic for cardiovascular disease?
A: Demonstrating efficacy typically involves a multi-faceted approach. This includes in vitro studies to confirm the therapeutic's ability to modulate complement activity, followed by in vivo studies in relevant animal models of cardiovascular disease. Key endpoints often include reductions in inflammatory markers, improved cardiac function, decreased tissue damage, and changes in disease progression. Ultimately, robust clinical trial data in human patients would be required to confirm safety and efficacy.
Creative Biolabs is your trusted partner for advancing complement system therapeutics in ophthalmology. Our comprehensive suite of services, from recombinant protein supply to advanced antibody engineering and functional assays, is designed to empower your research and accelerate the development of innovative treatments for debilitating eye diseases.
Featured Services
Feature Products
Reference
-
DeVaughn, Hunter et al. "Complement Immune System in Pulmonary Hypertension-Cooperating Roles of Circadian Rhythmicity in Complement-Mediated Vascular Pathology." International journal of molecular sciences vol. 25,23 12823. 28 Nov. 2024, DOI:10.3390/ijms252312823. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig.1 Autoimmune disorders and complement cascade initiation in PAH pathological development.1
Fig.1 Autoimmune disorders and complement cascade initiation in PAH pathological development.1