Accomplishing targeted drug delivery to precise cells or tissues stands as a pivotal hurdle in medical investigation, aimed at mitigating superfluous off-target repercussions. For the targeted conveyance of therapeutic or diagnostic agents, aptamers binding to cell surface molecules have emerged as versatile recognition ligands, boasting high specificity, minimal toxicity, and cellular internalization.
Creative Biolabs offers adept aptamer conjugation services, linking aptamers to diverse therapeutic agents or nanoparticles, thereby expanding their utility across the realm of treatment and diagnosis.
Background of Aptamer-Based Conjugation
The fundamental concept underlying therapeutic targeted drug delivery strategies involves the utilization of specific recognition mechanisms for afflicted cells as the designated ligands for therapeutic carriers, aiming to enhance treatment efficiency and tolerability. Within this context, the advancement of SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology has yielded intriguing molecular candidates known as aptamers, which intricately fold into distinctive three-dimensional configurations enabling precise binding to predefined target molecules.
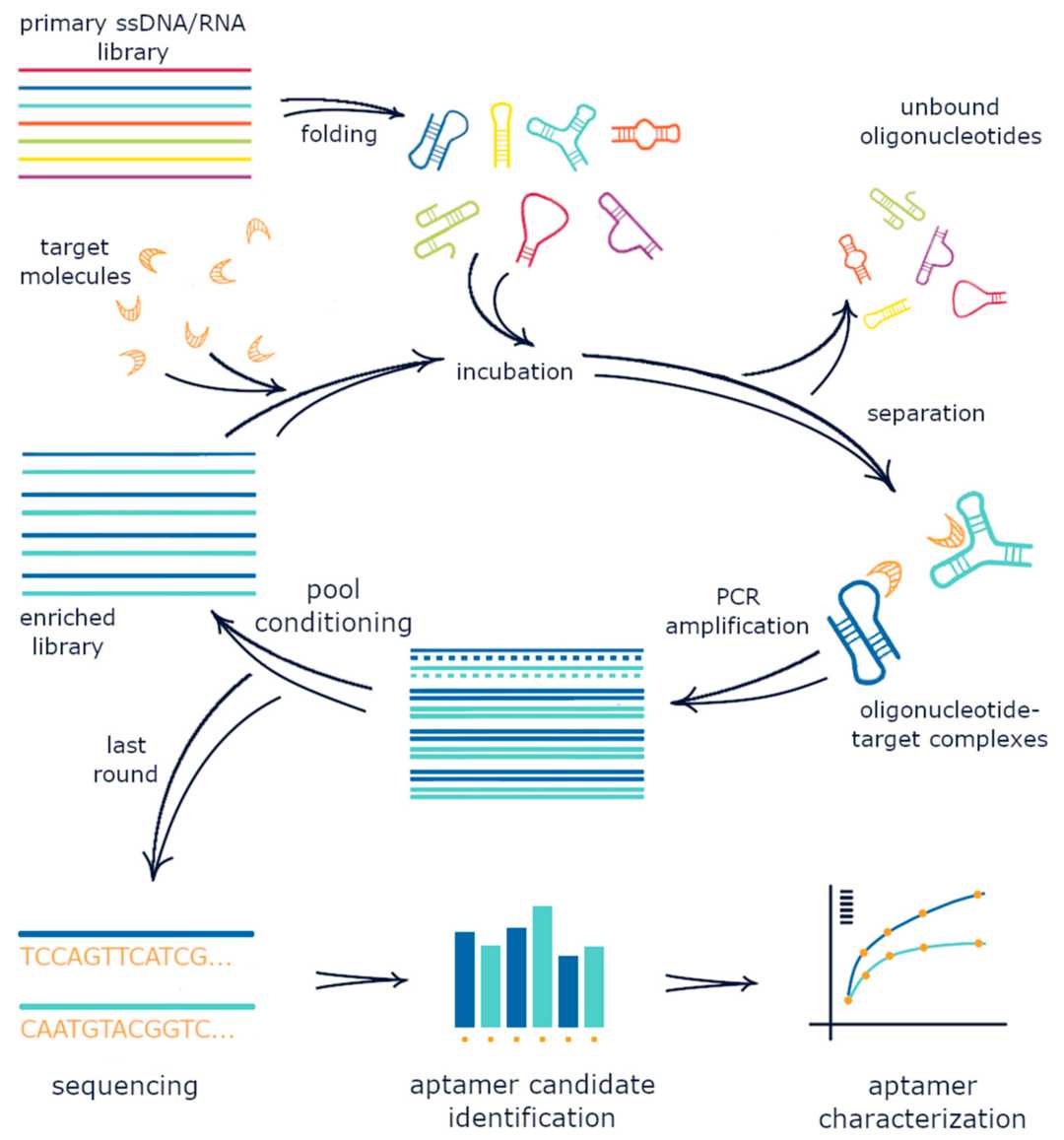 Fig. 1 Schematic representation of the SELEX process.1, 5.
Fig. 1 Schematic representation of the SELEX process.1, 5.
Aptamer-Based Conjugation Service
Based on the chemical properties of aptamers, the aptamer-based delivery strategy we developed is mainly divided into two different methods:
1. Aptamer-second reagent direct conjugation.
Aptamer-drug conjugation (ApDC) stands as a remarkably straightforward and effective paradigm, involving the direct non-covalent or covalent coupling of aptamer sequences with therapeutic agents.
Through facile non-covalent binding facilitated by aptamer incubation, a prime example lies in the context of retinoblastoma (RB) cells, wherein an RNA-based EpCAM aptamer, termed EpDT3, is combined with the cancer chemotherapeutic agent doxorubicin (Dox) to create a chimeric construct, EpDT3-Dox. This construct selectively delivers Dox to RB cells by targeting the epithelial cell adhesion molecule (EpCAM), inhibiting cell proliferation and achieving precise eradication of cancer stem cells within RB cells, thus circumventing the neurotoxicity associated with independent Dox delivery to non-cancerous neural cells. Covalent linkages between aptamers and drugs confer enhanced stability to binding interactions, rendering this approach more commonplace. Particularly advantageous for reagents incompatible with nucleic acid integration or those that would disrupt aptamer structure, the covalent conjugation of Dox and DNA aptamer sgc8c results in the sgc8c-Dox hybrid complex, which exhibits specific cytotoxicity against target CCRF-CEM (T-cell acute lymphoblastic leukemia, T-cell ALL) cells. This covalently conjugated complex demonstrates heightened molecular specificity compared to unconjugated Dox, underscoring its potential as a robust therapeutic agent.
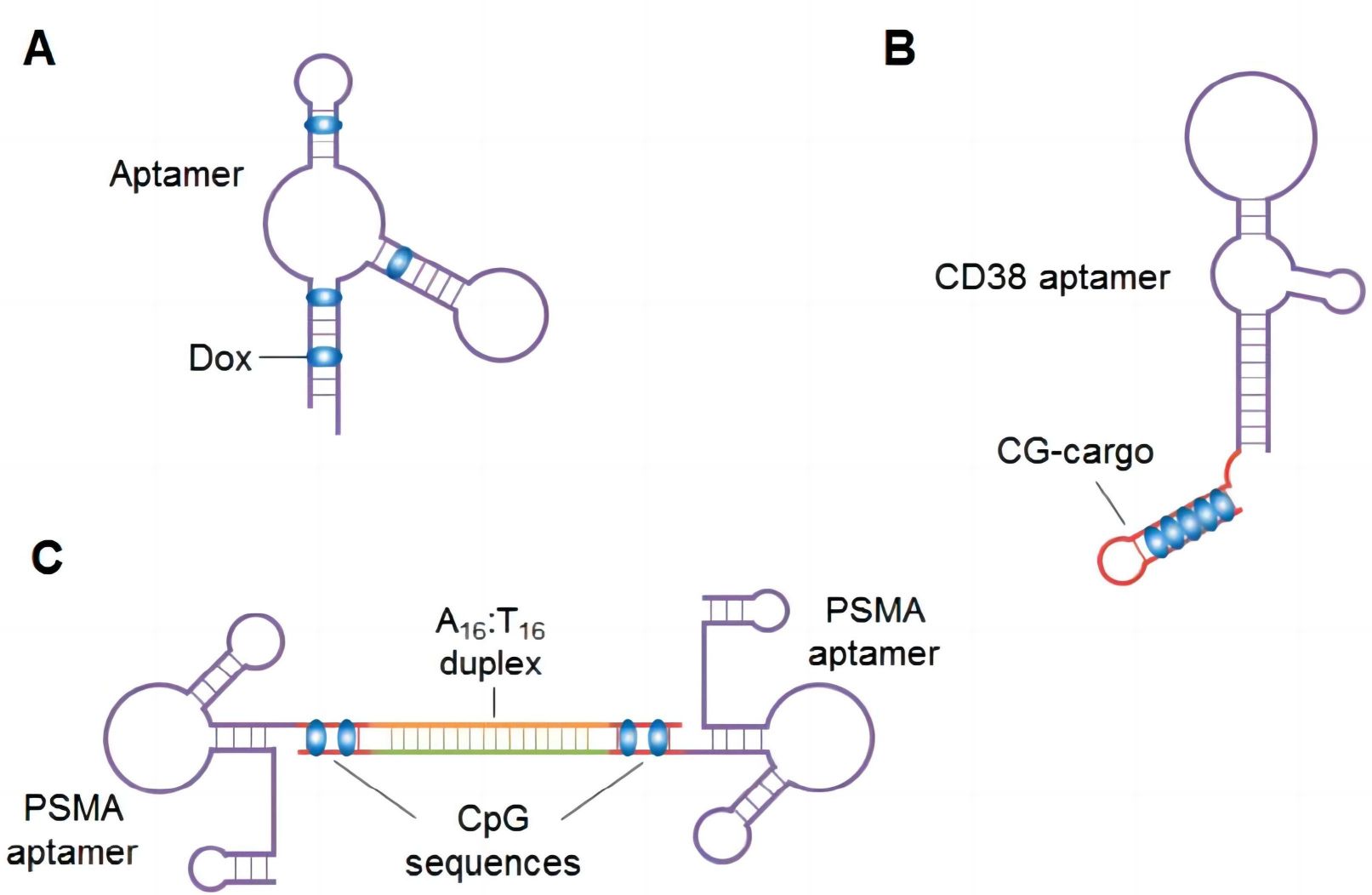 Fig. 2 Covalent and non-covalent conjugation between aptamers and drugs.2, 5
Fig. 2 Covalent and non-covalent conjugation between aptamers and drugs.2, 5
2. Aptamer-functionalization of nanomaterials.
While direct coupling of aptamers to drugs enhances the specificity of initially non-targeted therapeutics, the conjugation itself could influence drug functionality. To address these issues, cell-type-specific aptamers have been developed to decorate nanocarriers bearing diverse cargo. For instance, an aptamer-conjugated doxorubicin-loaded unimolecular micelle has been engineered. This carrier features a PSMA aptamer, a hydrophilic PEG shell, and a hyperbranched hydrophobic polyester core conjugated with Dox. Under mildly acidic conditions, drug release occurs alongside specific binding to target sites.
In a recent study, lipid nanoparticles (LNPs) were utilized as carriers. DNA aptamers targeting rat and human osteoblasts were employed to modify LNPs encapsulating bone-specific (pleckstrin homology domain-containing family O member 1, Plekho1) siRNA, resulting in a nucleic acid aptamer-LNP-siRNA system. This system facilitated selective siRNA uptake by osteoblasts in vivo, leading to osteoblast-specific Plekho1 gene silencing, thereby promoting bone formation and enhancing mechanical performance in osteoporotic animals.
Beyond the mentioned nanomaterials, a versatile array is available, including gold nanoparticles, iron oxide nanoparticles, liposomes, viral capsids, among others. These materials can be assembled with cell-type-specific aptamers to exert targeted effects, catering to diverse contextual requirements.
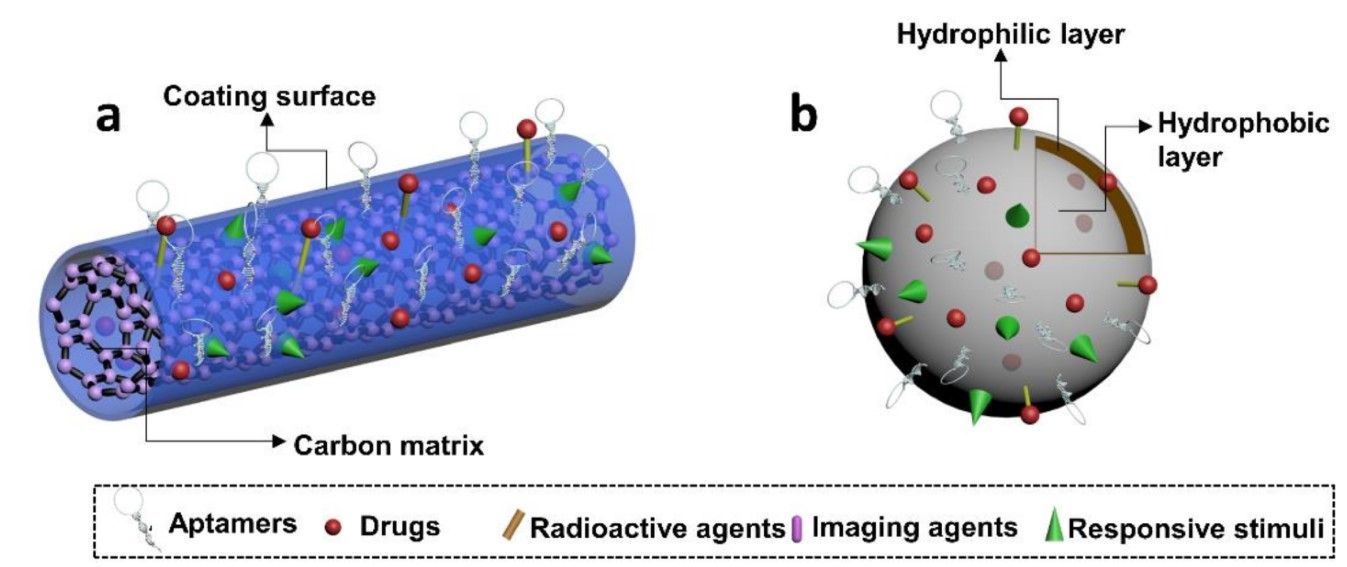 Fig. 3 Schematic illustration depicting aptamer-functionalized nanomaterials.3, 5
Fig. 3 Schematic illustration depicting aptamer-functionalized nanomaterials.3, 5
In both scenarios, aptamers are harnessed as targeting agents for the recognition of afflicted tissues, enabling precise engagement with therapeutic agents. This approach, which is both selective and economical, facilitates specific interventions. To date, aptamers have been either covalently or physically linked to therapeutic agents encompassing chemotherapeutics, toxins, and therapeutic oligonucleotides. Numerous strategies for aptamer functionalization have emerged as valuable techniques, augmenting nanoparticle specificity.
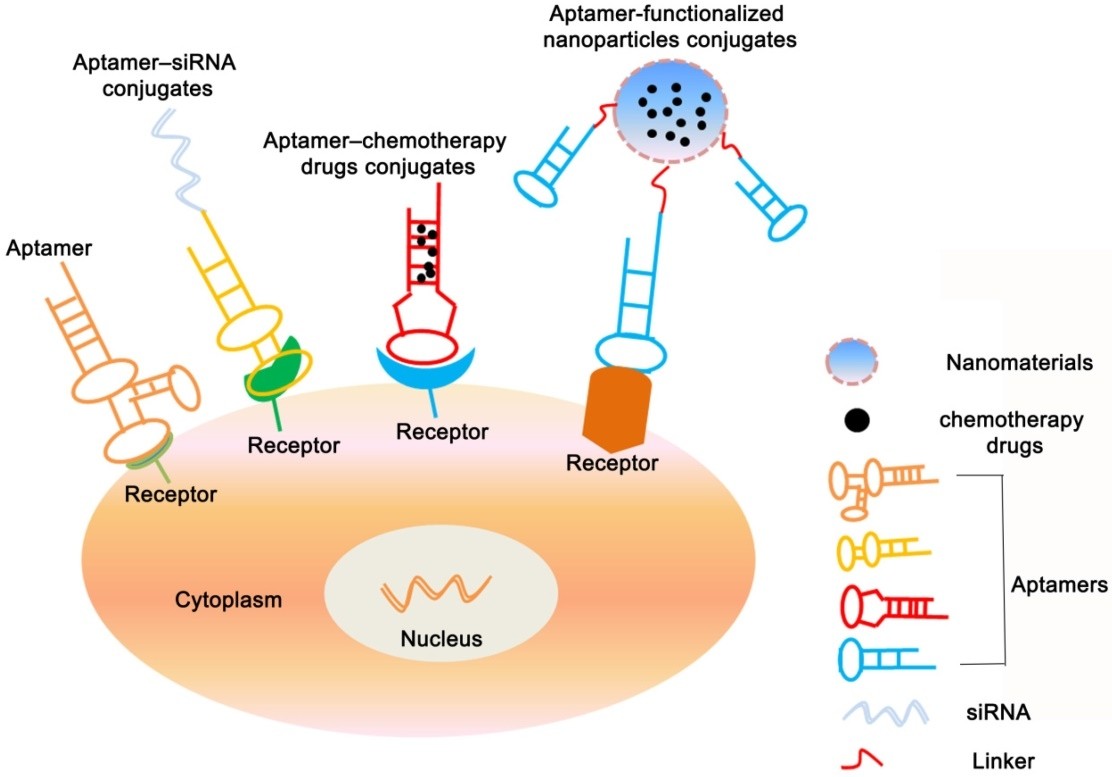 Fig. 4 Aptamer-based conjugates therapy.4, 5
Fig. 4 Aptamer-based conjugates therapy.4, 5
Aptamer-based Conjugation Services
Creative Biolabs offers mature services for aptamer-based conjugation services, please contact us for more details.
References
-
Komarova, Natalia, and Alexander Kuznetsov. "Inside the black box: what makes SELEX better?" Molecules 24.19 (2019): 3598.
-
Hori, Shin-ichiro, et al. "Current advances in aptamers for cancer diagnosis and therapy." Cancers 10.1 (2018): 9.
-
Liu, Mengping, et al. "Aptamer-enabled nanomaterials for therapeutics, drug targeting and imaging." Cells 11.1 (2022): 159.
-
Han, Jing, et al. "Application and development of aptamer in cancer: from clinical diagnosis to cancer therapy." Journal of Cancer 11.23 (2020): 6902.
-
under Open Access license CC BY 4.0, without modification.
Questions & Answer
A: Aptamers offer versatile conjugation opportunities to various molecules and materials, including proteins, nanoparticles, and surfaces, delivering benefits like high affinity, stability, and potential for targeted applications.
A: Aptamer-based conjugation finds widespread usage in in vitro investigations to elucidate molecular interactions, establish diagnostic assays, and enable precise molecule delivery to distinct cells or tissues. Although its predominant application remains in vitro, current research delves into its viability for in vivo purposes, encompassing targeted drug delivery and imaging. Yet, further studies and refinement are imperative to ensure secure and efficacious in vivo implementation.
A: Techniques such as gel electrophoresis, mass spectrometry, surface plasmon resonance (SPR), fluorescence spectroscopy, and atomic force microscopy (AFM) are often employed to analyze and characterize aptamer-based conjugates.
For Research Use Only.
Related Sections:

 Fig. 1 Schematic representation of the SELEX process.1, 5.
Fig. 1 Schematic representation of the SELEX process.1, 5.
 Fig. 2 Covalent and non-covalent conjugation between aptamers and drugs.2, 5
Fig. 2 Covalent and non-covalent conjugation between aptamers and drugs.2, 5
 Fig. 3 Schematic illustration depicting aptamer-functionalized nanomaterials.3, 5
Fig. 3 Schematic illustration depicting aptamer-functionalized nanomaterials.3, 5
 Fig. 4 Aptamer-based conjugates therapy.4, 5
Fig. 4 Aptamer-based conjugates therapy.4, 5