Introduction of Complement Component 4 (C4)
Complement C4 is one of the components in the intricate complement system, which is encoded by two closely linked genes (C4A and C4B) located within the major histocompatibility complex (MHC) on chromosome 6p21.3. Because the C4A-C4B genes are two-locus allelic model, the genetics of human complement C4 is characterized with an abundant variation in the gene copy number and gene size within a population.
It is documented that the size of C4A or C4B gene can be 21 kb (long, L) or 14.6 kb (short, S), and the encoded proteins share 99% identity and differ by four isotype-specific amino acids in exon 26. Among different healthy individuals, 2 to 7 copies of C4 genes in a diploid genome are frequently present, which may increase the diversity and reduce the possibility of a total deficiency of C4A and C4B proteins in one subject.
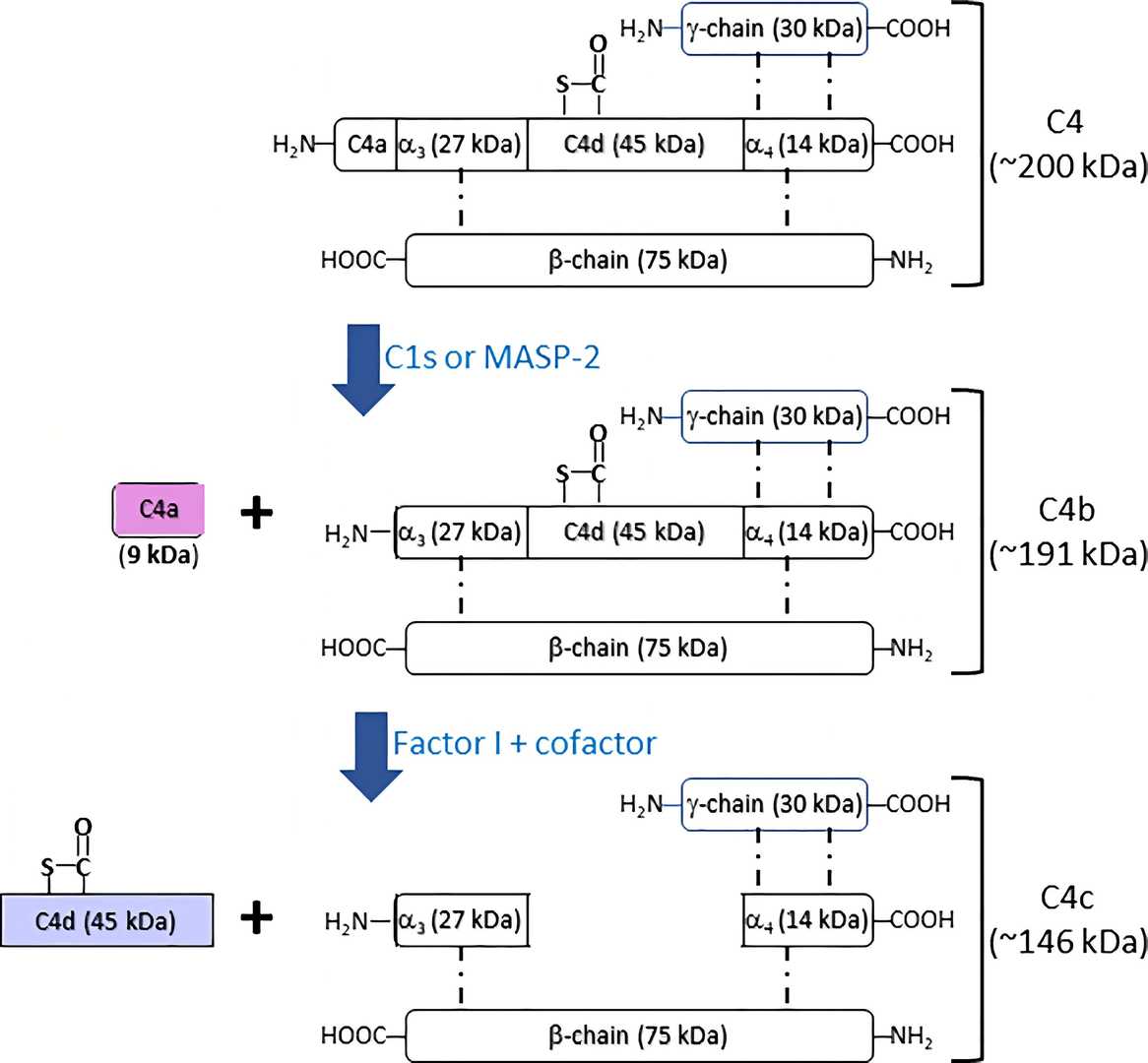
Fig. 1 Fragmentation of complement C4.1
Function of Complement Component 4
As an important complement component, C4 plays a number of critical functions in immunity, tolerance, and autoimmunity with the other numerous components. Additionally, it is significant that C4 can connect the recognition pathways of the overall system instigated by antibody-antigen (Ab-Ag) complexes to the other effector proteins of the innate immune response. In the activation of the complement pathway, the protease will cleave C4 into two parts, C4a and C4b. C4a is a small peptide (~9 kDa), which can be cleaved to release C4 anaphylatoxin to mediate the local inflammatory reaction. C4b is a surface-attached large protein (~190 kDa), which serves as a platform for the formation of C3 convertase (C4b2a) that is responsible for the activation of C3 and downstream enzymic reactions.
Clinical Significance
As a result of C4 deficiency, the patients have a remarkable decrease in serum opsonic, chemotactic, tolerogenic and bactericidal activities via activation of the classical pathway. It is reported that homozygous or heterozygous deficiency of C4A is increased in Complement Therapeutics for Systemic Lupus Erythematosus (SLE) patient cohorts compared with the healthy populations. C4B deficiency is closely associated with increased risk of IgA nephropathy, autoimmune conditions such as insulin-dependent diabetes, dermatological diseases, specifically Disseminated Lupus Erythematosus (DLE), angioedema, and urticaria. Strong evidence has revealed that homozygous C4B deficiency presents an increased prevalence in children with bacteremia and/or bacterial meningitis. Moreover, C4 has attracted massive attention for its role in schizophrenia risk and development.
Creative Biolabs offers a full range of complement therapeutic development services for the C5aR related disease. Please contact us for more information or a detailed quotation.
Published Data
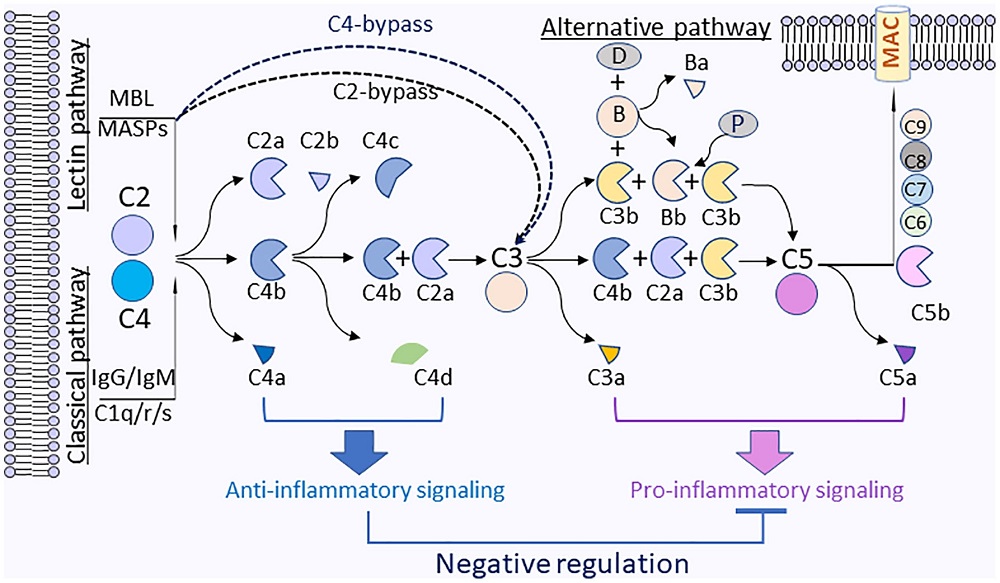 Fig.2 Complement C4 activation fragments C4a and C4d mediate anti-inflammatory effects.1
Fig.2 Complement C4 activation fragments C4a and C4d mediate anti-inflammatory effects.1
C4 plays a pivotal role in curbing human adenovirus infection through direct inactivation of the viral capsid. This process involves prompt C4 activation and cleavage deposition of C4b on the capsid, facilitated by antibodies via the classical complement pathway. C4b, once deposited, neutralizes the infection without C2 and C3 but necessitates C1q antibody involvement. It impedes capsid disassembly, thwarting endosomal escape and cytosolic entry. In C4-deficient mice, viral burdens increase. Moreover, complement acts synergistically with the Fc receptor TRIM21 to obstruct adenoviral gene therapy vector transduction. Altering complement activity may thwart virus infections and boost gene therapy effectiveness.
Reference
-
Wang, Hongbin, and Mengyao Liu. "Complement C4, infections, and autoimmune diseases." Frontiers in immunology 12 (2021): 694928. Distributed under Open Access license CC BY 4.0, without modification.
Related Product
Questions & Answer
A: In the context of neuroinflammatory diseases such as Alzheimer's disease, complement C4 emerges as a significant player in driving neurodegenerative processes. Dysregulated complement activation, including C4, contributes to synaptic loss, neuroinflammation, and amyloid-beta deposition. Targeting complement C4 holds promise in attenuating neuroinflammation and neuronal damage. C4-targeted therapies, by modulating neuroinflammatory responses, could potentially slow disease progression, offering a novel avenue for therapeutic intervention in neurodegenerative disorders.
A: Detailed insights into the three-dimensional structure of C4 molecules aid in the rational design of inhibitors, antibodies, and other biologics, optimizing their binding affinity and specificity.
A: Emerging research has shed light on the interactions between C4 isoforms, co-factors, and proteases, providing insights into the intricacies of C4 activation and its downstream effects.
For Research Use Only.
Related Sections:


 Fig.2 Complement C4 activation fragments C4a and C4d mediate anti-inflammatory effects.1
Fig.2 Complement C4 activation fragments C4a and C4d mediate anti-inflammatory effects.1