Immunopathology Complement Activation Complement Molecular Mechanisms Related Products Hot Services Q&A Resources
Are you currently facing challenges in developing effective therapies for Hemolytic Uremic Syndrome (HUS), including difficulties in targeting the complement system, complexities in patient stratification, and the need for personalized treatment strategies? Creative Biolabs’ innovative solutions help you accelerate your research and development of complement-targeted therapeutics for HUS through our comprehensive suite of cutting-edge tools and expertise, including advanced recombinant protein production, precise antibody development, and customized assay development.
HUS and Immunopathology
Hemolytic uremic syndrome (HUS) is a disorder characterized by the abrupt onset of acute hemolytic anemia, kidney failure (uremia), and thrombocytopenia, which is marked by reduced platelet levels in the blood. While the kidneys are primarily affected, other organs, including the brain and heart, may also suffer damage due to the involvement of small blood vessels. HUS mainly affects children younger than five years old and is the leading cause of acute kidney failure in this age bracket, though it is also increasingly identified in adults.
Symptoms of Hemolytic Uremic Syndrome
-
Thrombotic microangiopathy (TMA): abdominal pain, low platelet count, decreased haptoglobin, elevated lactate dehydrogenase LDH, anemia/schistocytes, proteinuria, decreased urination, pale skin, unexplained bruises or bleeding, fatigue, edema, nausea/vomiting, and bloody diarrhea.
-
Systemic symptoms: acute kidney failure, hypertension, myocardial infarction, stroke, lung complications, pancreatitis, liver necrosis, encephalopathy, seizure, and coma.
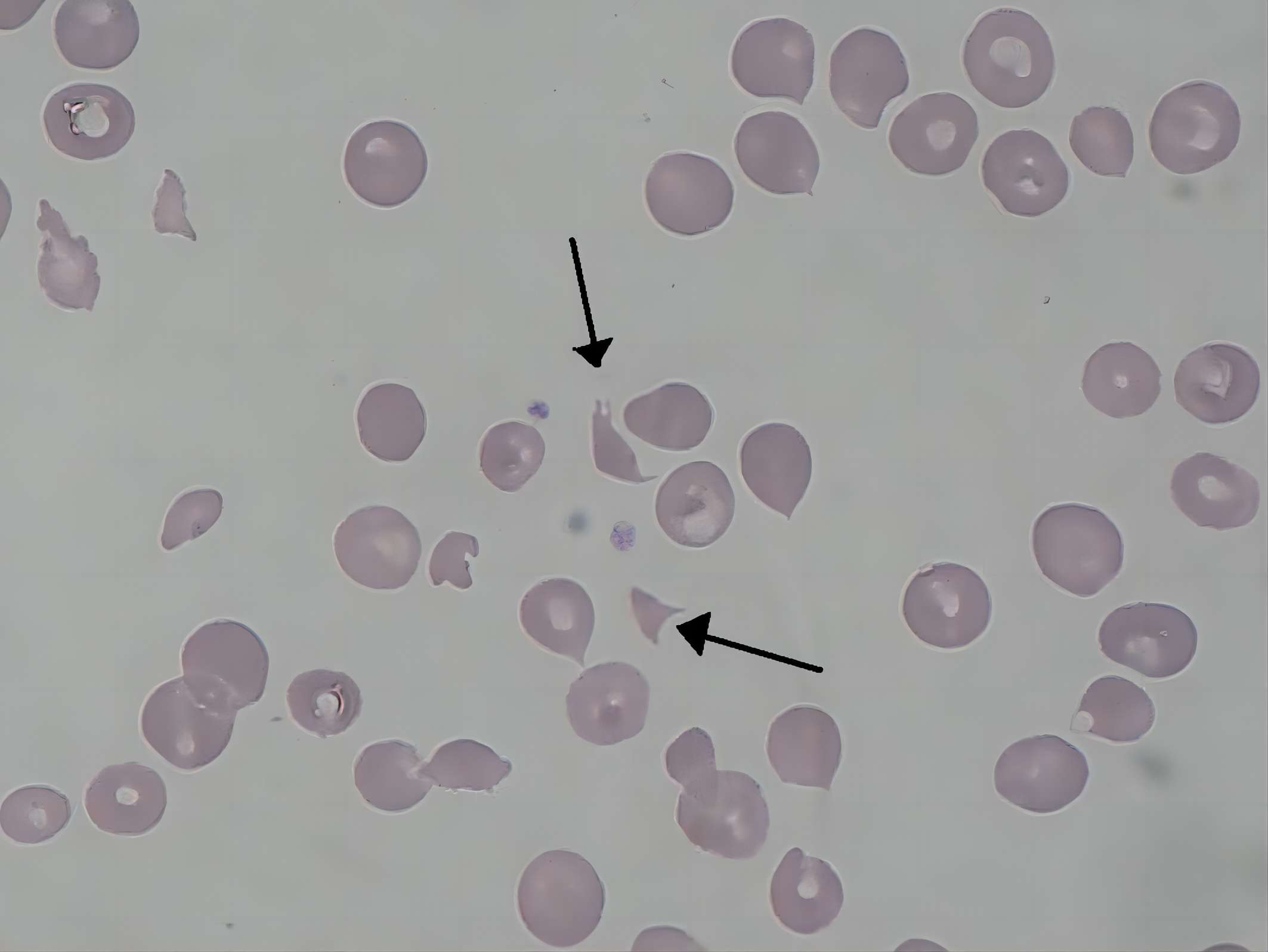
Fig. 1 Schizocytes in hemolytic-uremic syndrome.1
Causes of Hemolytic Uremic Syndrome
HUS can be classified as typical and atypical. Typical HUS is often caused by infections of the gastrointestinal tract, associated with gastrointestinal symptoms, including vomiting and diarrhea. Atypical HUS is not associated with an infection of the digestive tract but has close relationship with several genetic mutations that cause chronic, uncontrolled, and excessive activation of complement.
Typical HUS: It is primarily caused by infection with Shiga toxin-producing E. coli strains, particularly serotypes O157:H7 and O104:H4, among others. This infection can result in kidney damage either by direct cellular injury or by obstruction from lysed blood cells, and recent research also indicates viral involvement.
Atypical HUS: It may occur sporadically or heritably, associated with genetic anomalies in complement regulatory proteins like C3, factor B, factor H, factor I, and CD46. Shiga toxin directly activates the complement's alternative pathway and disrupts regulation by binding to factor H, highlighting its significance.
Complement Activation Pathways and HUS Pathogenesis
The complement system is integral to the pathogenesis of aHUS, functioning as a complex network of proteins that collaborate to clear pathogens and damaged cells, and can be activated via three main pathways:
Table 1 Complement activation pathways in HUS.
|
Complement Activation Pathways
|
Description
|
|
Classical Pathway
|
Activated by antigen-antibody complexes. This pathway is activated when antibodies attach to antigens, creating immune complexes that subsequently initiate a series of protein interactions. This pathway plays a significant role in clearing infections with encapsulated bacteria and in the pathogenesis of autoimmune diseases.
|
|
Alternative Pathway
|
Triggered by the spontaneous hydrolysis of C3 or specific microbial surfaces, the alternative pathway, in contrast to the classical pathway, maintains a continuous low-level activation. Spontaneous hydrolysis of C3 initiates a cascade involving factors B, D, and properdin. This pathway can be amplified by certain microbial surfaces, such as those of bacteria and viruses, leading to rapid complement activation in the absence of antibodies. It is crucial for early defense against pathogens and is often the primary pathway involved in aHUS.
|
|
Lectin Pathway
|
Activated by MBL binding to carbohydrates on microbial surfaces. Analogous to the classical pathway, the lectin pathway is activated through the recognition of distinct molecular patterns on pathogens. This binding activates MASPs, which then activate downstream complement components. The lectin pathway provides another antibody-independent mechanism for complement activation and plays a vital role in the innate immune response to a variety of microorganisms.
|
Complement Molecular Mechanisms in HUS
In aHUS, complement system dysregulation—especially within the alternative pathway—results in excessive activation that damages endothelial cells lining blood vessels, predominantly in the kidneys, leading to thrombotic microangiopathy (TMA), a hallmark of HUS. Key complement proteins involved include:
Table 2 Molecular mechanisms of complement-mediated.
|
Key Complement Components
|
Functions
|
|
C3
|
In aHUS, C3 activity is often abnormally elevated due to the lack of proper regulation by CFH, CFI, or MCP. This leads to a vicious cycle of uncontrolled complement activation and endothelial damage.
|
|
Complement Factor B (CFB)
|
A serine protease that, when activated, forms the C3 convertase (C3bBb) with C3b, a crucial enzyme in the alternative pathway that amplifies complement activation. In aHUS, CFB contributes to the uncontrolled amplification of the alternative pathway.
|
|
Complement Factor H (CFH)
|
A crucial regulator of the alternative complement pathway prevents excessive activation by promoting the decay of C3 convertase (C3bBb) and serving as a cofactor for Factor I-mediated C3b cleavage; mutations in the CFH gene can lead to deficient or dysfunctional CFH protein, causing unregulated complement activation and increasing the risk of aHUS, with severity varying by mutation type.
|
|
Complement Factor I (CFI)
|
CFI is a key serine protease in the complement system that, with cofactors such as CFH, cleaves and inactivates C3b and C4b to restrict complement activation amplification; mutations in the CFI gene can hinder this regulatory function, promoting aHUS development, with varying degrees of complement dysregulation depending on the specific mutation.
|
|
Membrane Cofactor Protein (MCP or CD46)
|
MCP is a cell-surface protein that regulates complement activation by acting as a cofactor for Factor I-mediated inactivation of C3b and C4b on cell surfaces, expressed on various cell types like endothelial cells, platelets, and leukocytes, thereby protecting host cells from complement-induced damage; mutations in the MCP gene can compromise this protective role, increasing susceptibility to complement-mediated injury in aHUS.
|
Creative Biolabs has established advanced Complement Therapeutics platform including antibody engineering platform, protease inhibitor platform, and drug discovery platform, and is equipped to offer a full range of biotherapeutics development services regarding drug discovery and validation for HUS. Please contact us for detailed information.
Related Hot Products
Our comprehensive complement platform offers a broad and cost-effective range of complement-associated products. For further details, please do not hesitate to contact us.
Table 3 Featured products.
Related Hot Services
Table 4 Complement test services for SS-related complement studies.
Resources
Reference
-
From Wikipedia: By Paulo Henrique Orlandi Mourao - Own work, CC BY-SA 3.0 https://commons.wikimedia.org/wiki/File:Schizocyte_smear_2009-12-22.JPG
For Research Use Only.
Related Sections: