RA and Immunopathology Complement Activation in RA Complement Molecular Mechanisms Complement Test Services
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disorder that primarily affects synovial joints, leading to inflammation, cartilage degradation, and bone erosion. While various immunological pathways are involved in RA pathogenesis, the complement system has emerged as a key player in the amplification of inflammatory responses. At Creative Biolabs, we are committed to supporting RA research through our specialized complement system analysis and custom assay development services.
Rheumatoid Arthritis and Immunopathology
RA is a debilitating autoimmune disorder characterized by persistent inflammation of the synovial joints. Unlike osteoarthritis, which is mechanical and degenerative in nature, RA arises from a dysregulated immune system that mistakenly attacks the body's own tissues—primarily the synovium, the lining of joints. This self-perpetuating inflammatory cycle leads to progressive joint damage, severe disability, and a significantly reduced quality of life if left unmanaged.
Immunopathological Features
The pathogenesis of RA is multifactorial, involving genetic predisposition (e.g., HLA-DR4), environmental triggers (e.g., smoking), and immunological abnormalities. Central to its progression is an aberrant immune response that includes both adaptive and innate components.
Key immunopathogenic processes include:
-
Activation of autoreactive T cells, particularly Th1 and Th17 subsets, which drive cytokine release and macrophage activation.
-
Autoantibody production by B cells, including rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies.
-
Synovial hyperplasia, forming a pannus that invades cartilage and bone.
-
Angiogenesis, enhancing leukocyte infiltration and perpetuating inflammation.
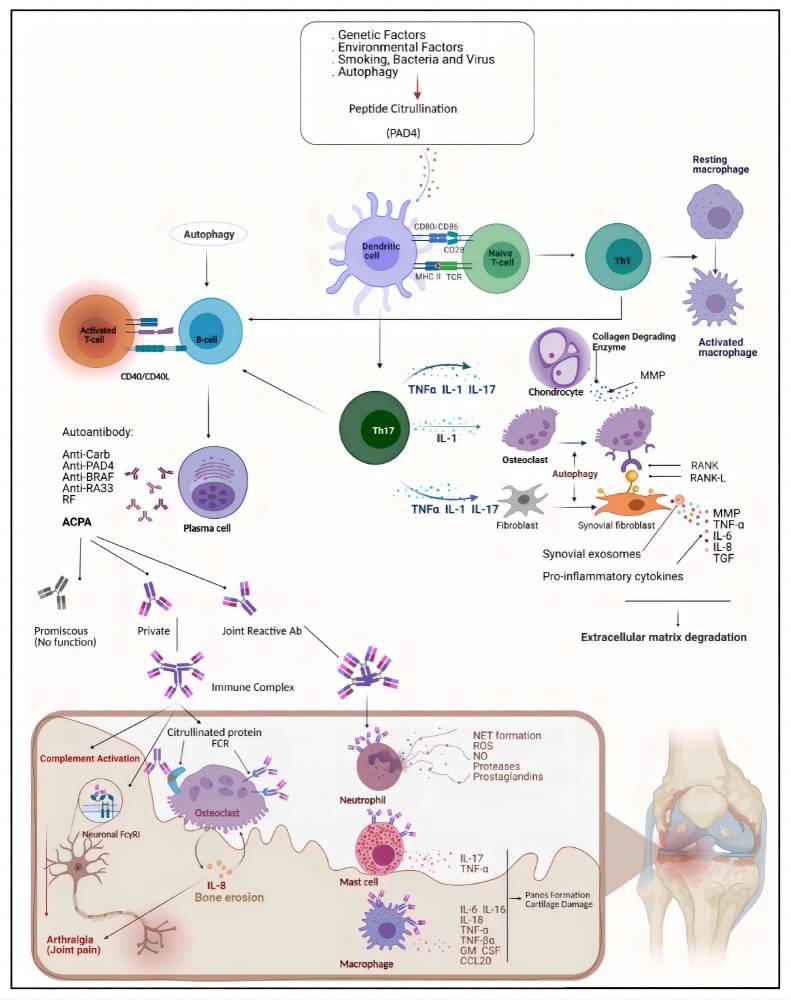 Fig. 1 Structure of C1 inhibitors.1,2
Fig. 1 Structure of C1 inhibitors.1,2
While much emphasis is placed on adaptive immunity in RA, the innate immune system serves as the initial amplifier of inflammation. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and, critically, the complement cascade work in concert to detect danger signals and trigger rapid pro-inflammatory responses. Increasing evidence indicates that early complement activation is not merely a consequence, but a driver of RA pathogenesis, making it a compelling target for both diagnostic and therapeutic intervention.
Complement Activation Pathways and RA Pathogenesis
The complement system is activated through three major pathways: the classical pathway, the alternative pathway, and the lectin pathway. In RA, all of these pathways may be involved in the pathologic process, but the alternative pathway and lectin pathway are thought to be important in the initiation and extension of the disease.
Table 1 Complement activation pathways and RA pathogenesis.
|
Complement Activation Pathways
|
Description
|
RA Pathogenesis
|
|
Classical Pathway
|
The classical pathway activates the C1q protein through antigen-antibody complexes.
|
In the synovial fluid of RA patients, large amounts of IgG and IgM bind to C1q, leading to a complement cascade reaction. This activation process further exacerbates synovial inflammation and results in the formation of the terminal complement complex (MAC), which causes irreversible damage to articular cartilage.
|
|
Alternative Pathway
|
The alternative pathway is initiated by direct spontaneous hydrolysis of C3 and continuously activates and amplifies the inflammatory response.
|
In RA, it has been found that C3d and Ba levels are significantly elevated in the synovial fluid of RA patients, suggesting that the alternative pathway is strongly activated in the local joint environment.
|
|
Lectin Pathway
|
The lectin pathway is initiated by recognizing glycosylation patterns on the surface of pathogenic microorganisms.
|
Although its exact mechanism has not been fully elucidated, experimental models suggest that this pathway may play an important role in the early stages of RA.
|
Molecular Mechanisms of Complement-Mediated
Complement activation plays a multifaceted role in the pathophysiology of RA by triggering a series of inflammatory events that maintain synovitis and promote tissue destruction. A large body of evidence suggests that complement activation occurs at both systemic and local (synovial) levels in RA patients.
-
Levels of complement activation products such as C3a, C5a and soluble membrane attack complexes (sC5b-9) are markedly elevated in the synovial fluid of rheumatoid arthritis.
-
Immunohistochemical analyses showed substantial deposition of complement components, including C1q, C3d, and C4d, in the inflamed synovial lining.
Table 2 Molecular mechanisms of complement-mediated.
|
Molecule
|
Function
|
|
C3a/C5a
|
-
Acts as a potent chemoattractant to recruit neutrophils, macrophages and mast cells to inflamed joints.
-
Promotes the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) from macrophages and fibroblast-like synoviocytes.
-
Induces vascular permeability and edema, exacerbating synovial swelling.
|
|
MAC/C5b-9
|
-
Forms pores in the cell membranes of chondrocytes and synoviocytes, leading to sub-soluble or lysogenic cell damage.
-
Even at sub-soluble concentrations, MAC triggers the release of matrix metalloproteinase (MMP), which degrades the cartilage extracellular matrix.
-
Induces oxidative stress, further exacerbating tissue damage.
|
|
Complement-conditioned immune complexes
|
-
RA is associated with high titers of circulating immune complexes (e.g., anti-CCP or RF-binding antigens) that activate the classical pathway via C1q binding.
-
Complement-coated immune complexes are deposited in the synovium, leading to impaired phagocytosis and localized inflammation.
|
Given the critical role of the complement system in RA, targeting its inhibition has significant potential. Several strategies are currently under development.
At Creative Biolabs, we provide researchers with sensitive, validated assays to detect and quantify complement components and activation products across multiple sample types (serum, synovial fluid, tissue biopsies), enabling a comprehensive evaluation of complement involvement in RA.
Complement Test Services at Creative Biolabs
We offer a wide range of services to support RA-related complement studies.
Table 3 Complement test services for RA-related complement studies.
All assays are designed for research use only, and optimized for high specificity, sensitivity, and reproducibility. The role of the complement system in rheumatoid arthritis is now recognized as central to its pathogenesis and progression. Targeted complement analysis can provide valuable insights into:
-
Disease mechanisms
-
Biomarker discovery
-
Treatment assessment
We are proud to empower RA researchers with advanced complement profiling tools and tailored assay development solutions. By translating complex immunology into actionable results, we aim to accelerate your discoveries in autoimmune disease research. If you want more information, please feel free to contact us.
References
-
Mueller, Anna-Lena, et al. "Recent advances in understanding the pathogenesis of rheumatoid arthritis: new treatment strategies." Cells 10.11 (2021): 3017. https://doi.org/10.3390/cells10113017
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig. 1 Structure of C1 inhibitors.1,2
Fig. 1 Structure of C1 inhibitors.1,2