Introduction Workflow DeliverablesCase StudyWhy Choose us?Published Data FAQ Related Services Featured Products
Introduction
Complement-Dependent Cytotoxicity (CDC) is a critical immune mechanism by which therapeutic antibodies eliminate target cells. Upon binding to its antigen, an antibody can activate the classical complement pathway, leading to the formation of the Membrane Attack Complex (MAC) and subsequent cell lysis. This lytic mechanism is crucial for the efficacy of monoclonal antibodies used in treating cancers and autoimmune diseases, as shown in numerous studies. A robust and sensitive CDC assay is essential for the preclinical validation of a therapeutic antibody's mechanism of action.
Complement Fixation Assay Workflow
Samples Required
-
Therapeutic antibodies, including monoclonal and bispecific antibodies (mAbs, BsAbs).
-
Target cells (e.g., patient-derived primary cells, tumor cell lines, or engineered cell lines).
-
Recombinant proteins or peptides for positive controls.
Key Steps Involved
01
Project Scoping & Assay Design: We collaborate with you to define your research goals, select the optimal target cell line, and choose a quality-controlled complement source.
02
Sample Preparation & Assay Setup: Our expert team cultures the chosen target cells and prepares them for the assay. Your therapeutic antibodies and controls are then titrated and combined with the target cells and the selected complement source.
03
Lysis Induction & Data Acquisition: The reaction is incubated under optimal conditions to allow complement activation and subsequent cell lysis. We employ advanced non-radioactive readouts to measure cell death with high sensitivity.
04
Data Analysis & Quality Control: The raw data is meticulously analyzed to generate dose-response curves, calculate key metrics, and ensure all quality control parameters are met for reproducibility and accuracy.
05
Reporting & Consultation: You will receive a comprehensive final report containing all raw data files, a summary of statistical analyses, and a detailed description of the experimental methodology. Our scientists are available for a follow-up consultation to discuss the results and provide recommendations for future studies.
Estimated Timeframe The typical timeframe for this service ranges from 2 to 4 weeks, depending on the complexity of the project. Factors that may influence the duration include the number of antibodies to be tested, the type of cell lines used, and any specific custom assay requirements.
Deliverables
-
A comprehensive project report including a description of the experimental methodology.
-
Statistical analysis summary (e.g., EC50 values, maximal lysis percentages) and all raw data files.
-
Dose-response curves and graphs suitable for presentations and publications.
-
Detailed quality control data and a summary of assay validation.
Case Study
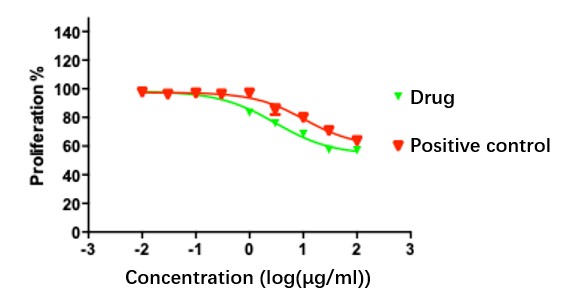 Fig.1 CDC results in Creative Biolabs.
Fig.1 CDC results in Creative Biolabs.
Creative Biolabs conducted a CDC assay to evaluate the efficacy of a monoclonal antibody. The test was performed on multiple cancer cell lines with a positive control included. Target cells were cultured in specific media, followed by serial dilution of the antibody and co-incubation with human serum. Luminescence was measured to assess cell viability. Data analysis demonstrated variable CDC effects across the different cell lines. This assay provides crucial insights into antibody-mediated cytotoxicity, assisting in the development of targeted immune therapies.
Why Choose Us?
At Creative Biolabs, we don't just provide a service; we become your partner in drug development. Our expert team offers a truly one-stop solution, giving you the clarity and confidence needed to make critical decisions.
Integrated Solutions:
A truly one-stop solution, integrating our CDC assay with complementary effector function tests (ADCC, ADCP) to provide a complete profile of your therapeutic agent.
Customization:
We specialize in customizing assays to account for crucial variables like antibody isotype and complement regulatory protein expression, ensuring the data is highly relevant.
Advanced Platforms:
We use advanced, non-radioactive platforms that ensure high-throughput, superior sensitivity, and rapid, reproducible results.
Published Data
A recent study published demonstrated the critical role of the CDC assay to validate the efficacy of an MUC1-targeted cancer vaccine. Specifically, the CDC Assay was employed to assess the Antibody Effector Function. The result confirmed that the vaccine-induced serum antibodies successfully caused potent cell lysis and killing of the MUC1-positive target cells, demonstrating the therapeutic agent's mechanism of action.
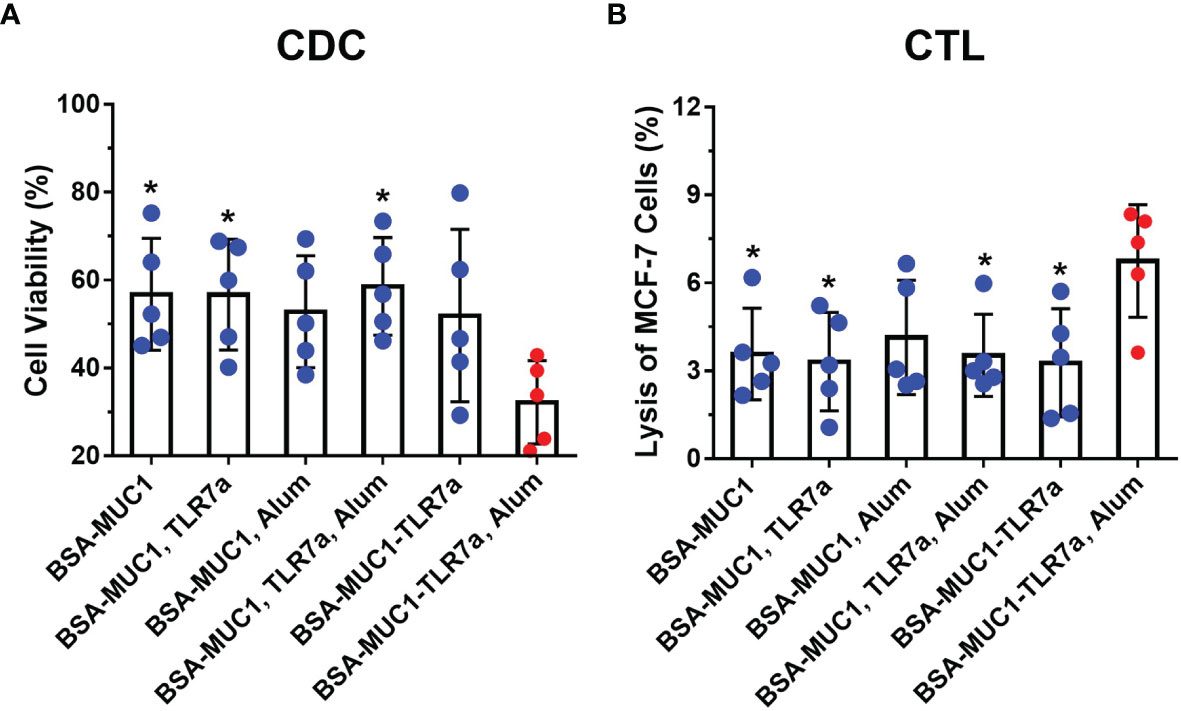 Fig.2 CDC assay of sera from vaccinated mice against MCF-7 cells.1
Fig.2 CDC assay of sera from vaccinated mice against MCF-7 cells.1
Frequently Asked Questions
A: Yes. Our team has extensive experience in adapting our CDC platform to accommodate a wide range of therapeutic formats, including bispecific antibodies, antibody-drug conjugates, and multi-domain proteins. We work closely with you to design a custom assay that accurately reflects your molecule's unique mechanism of action.
A: Our assays are performed with strict quality controls and validated protocols. We carefully optimize all parameters, including complement source, cell line viability, and antibody concentration, to minimize variability and ensure the highest data quality. All our reports include detailed quality control data.
A: Our complimentary consultation service is designed to help you with this exact question. We will discuss your target species and therapeutic goals to recommend the most suitable complement source, whether it's human, rabbit, or a non-rodent serum, ensuring the assay is physiologically relevant.
A: Absolutely. Our high-throughput, miniaturized formats are ideal for early-stage lead screening and candidate optimization, allowing you to quickly and cost-effectively screen a large number of molecules to identify those with potent CDC activity.
Related Services
Creative Biolabs also offers a range of Complement Test Services, Which can further support your research in the complement system.
Featured Products
The depth of your preclinical data is a direct reflection of your molecule’s potential. Creative Biolabs' Complement CDC Assay Service provides the expert platform and one-on-one consultation you need to build a robust data package. We deliver high-sensitivity, reliable results that will empower your drug discovery efforts and accelerate your journey to the clinic. For detailed information and to discuss your project, please contact us.
Reference
-
Zhou, Shi-Hao, et al. "Alum adjuvant and built-in TLR7 agonist synergistically enhance anti-MUC1 immune responses for cancer vaccine." Frontiers in Immunology 13 (2022): 857779. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3389/fimmu.2022.857779
For Research Use Only.
Related Sections:

 Fig.1 CDC results in Creative Biolabs.
Fig.1 CDC results in Creative Biolabs.
 Fig.2 CDC assay of sera from vaccinated mice against MCF-7 cells.1
Fig.2 CDC assay of sera from vaccinated mice against MCF-7 cells.1