Introduction What We Can Offer? Why Choose Us? Published Data FAQs Related Products Services
Accelerate Your Research and Development!
Are you currently facing challenges in developing targeted therapies for complement-mediated diseases or struggling with uncontrolled inflammation? Our Creative Biolabs Complement Therapeutic Target-C1 Inhibitor solutions help you accelerate therapeutic development and achieve precise complement regulation through advanced protein engineering and innovative antibody development techniques, ensuring high-quality, effective modulators for your critical projects.
Contact our team to get an inquiry now!
Introduction
C1-inhibitor (C1-INH) constitutes a central regulatory protein in the human complement system, a vital element of innate immunity. As a member of the serpin (serine protease inhibitor) superfamily, C1-INH serves as the primary endogenous inhibitor of the classical and lectin pathways of complement activation. Its fundamental role involves preventing uncontrolled activation and subsequent tissue damage by inactivating key proteases such as C1r, C1s, mannan-binding lectin-associated serine proteases (MASP-1 and MASP-2), Factor XIIa, and kallikrein. This broad inhibitory spectrum also extends to the contact system, linking complement regulation with coagulation and fibrinolysis pathways.
Structurally, C1-INH is a single-chain glycoprotein featuring a reactive center loop that functions as a pseudosubstrate for targeted proteases. Upon binding, it undergoes conformational rearrangement, irreversibly capturing the protease and inactivating it. This sophisticated mechanism critically regulates immune equilibrium. Functional impairment or deficiency of C1-INH provokes severe pathologies, predominantly hereditary angioedema (HAE) and acquired angioedema (AAE), distinguished by recurrent swelling episodes. Beyond angioedema, C1-INH plays a protective role in various inflammatory and autoimmune diseases, including sepsis, ischemia-reperfusion injury, rheumatoid arthritis, and systemic lupus erythematosus, by dampening excessive complement activation and inflammation. The therapeutic potential of C1-INH modulation is increasingly recognized, offering promising avenues for treating a spectrum of complement-mediated disorders.
 Distributed under Open Access license CC BY-SA 3.0, from Wiki,
without modification.
Distributed under Open Access license CC BY-SA 3.0, from Wiki,
without modification.
Fig.1 Structure of C1-inhibitor.
C1 Inhibitor Related Disease
C1-inhibitor deficiency correlates with hereditary angioedema (HAE) and acquired angioedema (AAE), both triggering recurrent, potentially fatal swelling episodes.
-
Hereditary Angioedema (HAE)
HAE causes recurring severe edema attacks, typically affecting limbs, face, and digestive tract. This condition stems from heterozygous C1-inhibitor deficiency. Documented mutations cause diminished plasma C1-inhibitor concentrations or generate dysfunctional C1-inhibitor proteins.
-
Acquired Angioedema (AAE)
First recognized in 1972, AAE entails acquired C1-inhibitor deficiency and classical complement pathway hyperactivation. Edema recurrence remains unpredictable, persisting 2-5 days with disfiguring, non-itchy skin swelling. Affected regions may include facial tissues, extremities, genitalia, buttocks, abdomen, bowels, or bladder.
C1-Inhibitor Related Therapeutics
Currently, C1-inhibitor is the only complement-focused protease inhibitor marketed commercially. C1-inhibitor formulations have served as hereditary angioedema (HAE) prophylaxis for years in Europe. This inhibitor targets not only C1 but also other complement cascade proteases, including MASP-2, plasmin, thrombin, factor Xa, and contact pathways.
-
rhC1INH
Recombinant human C1-inhibitor (rhC1INH) (Pharming Group N.Y) counters deficiency-induced edema. During edema management trials, 190 participants underwent 714 intravenous rhC1INH administrations. Outcomes demonstrated efficacy against all HAE attack localizations. Safety assessments encompassing 300 rhC1INH doses in healthy volunteers or HAE patients revealed no detectable anti-rhC1INH autoantibodies within 90 days.
Creative Biolabs delivers comprehensive or modular solutions personalized to client specifications. If you are interested in our platform or you are calling for our services, please contact us.
What We Can Offer?
Creative Biolabs provides a complete portfolio for C1-inhibitor therapy R&D assistance:
-
Recombinant C1-Inhibitor Proteins: High-purity C1-INH for research, assay development, and drug screening.
-
Anti-C1-Inhibitor Antibodies: Specific monoclonal and polyclonal antibodies for detection and research.
-
C1-Inhibitor Activity Assay Kits: Kits for accurate C1-INH functional activity measurement.
-
Custom C1-Inhibitor Protein Expression & Purification: Tailored services for C1-INH variant/mimetic expression and purification.
-
C1-Inhibitor Modulator Screening Services: High-throughput platforms identifying novel C1-INH modulators (small molecules/biologics).
-
Antibody Development Services: Comprehensive therapeutic antibody discovery/engineering targeting or mimicking C1-INH.
-
Complement Pathway Functional Assays: Assays assessing C1-INH modulator impact on classical, lectin, and alternative complement pathways.
Why Choose Us?
Creative Biolabs provides unique strengths in C1-inhibitor therapy development, integrating profound proficiency in complement biology and protein engineering with cutting-edge high-throughput screening platforms and novel protein production technologies.
Key Advantages:
-
Unparalleled Expertise: Decades of specialized experience in complement system biology and therapeutic development.
-
Cutting-Edge Platforms: Advanced high-throughput screening and protein engineering technologies for rapid discovery.
-
Customized Solutions: Tailored experimental designs and flexible service models to meet unique project requirements.
-
Rigorous Quality Control: Comprehensive validation and robust quality assurance protocols for reliable results.
-
Accelerated Timelines: Efficient workflows designed to significantly reduce your drug discovery timelines.
-
Proven Track Record: Demonstrated success and Published Data in developing effective complement modulators.
Access the Creative Biolabs Edge - Request a Proposal Now
Published Data
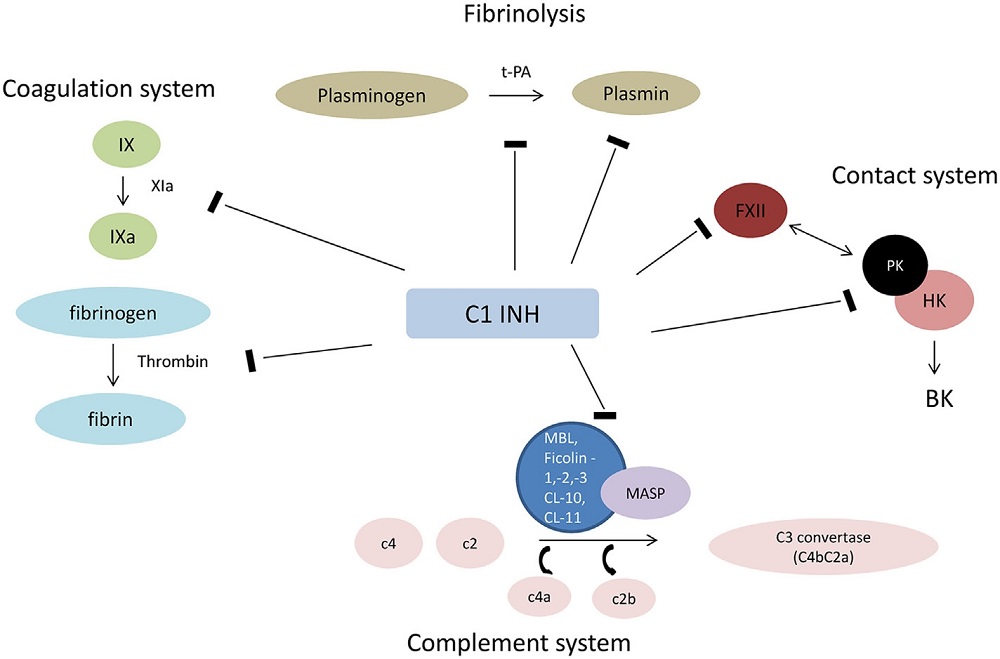 Fig.2 Engagement of C1INH with plasma cascades.1
Fig.2 Engagement of C1INH with plasma cascades.1
Acute myocardial infarction (AMI) persists as a significant contributor to contemporary morbidity and mortality. In model studies of myocardial ischemia-reperfusion (I/R) damage, the complement system, especially the lectin pathway, has been identified as a significant factor. The natural blocker of this pathway, C1 esterase inhibitor (C1INH), has demonstrated notable reduction in myocardial I/R injury. However, the role of lectin pathway activation in exacerbating myocardial I/R injury lacks confirmation in humans, questioning strategies focused solely on this pathway. C1INH’s ability to target multiple systems, including contact and coagulation, may offer broader therapeutic benefits.
FAQs
Q: How can modulating C1-inhibitor activity impact different disease states?
A: Modulating its activity can have profound effects across various diseases. Increasing its activity or providing functional mimetics can help control excessive complement activation and inflammation, which is beneficial in conditions like hereditary angioedema, sepsis, and ischemia-reperfusion injury. Conversely, in situations where complement activation is desired for therapeutic effect, such as certain anti-cancer strategies, transient inhibition might be explored. The specific impact depends on the precise mechanism of modulation and the disease context.
Q: What are the primary considerations when designing a therapeutic agent that targets C1-inhibitor?
A: Key considerations include ensuring high specificity for C1-inhibitor to minimize off-target effects, optimizing its potency for effective modulation, and designing for appropriate pharmacokinetic and pharmacodynamic profiles. Additionally, understanding the precise binding site and mechanism of action is crucial for predictable therapeutic outcomes. Safety and immunogenicity are also paramount, especially for biologic agents.
Q: Are there different approaches to targeting C1-inhibitor, and how do they compare?
A: Yes, approaches vary. One common strategy involves administering recombinant C1-inhibitor or its mimetics to supplement deficient levels or enhance inhibitory capacity. Another involves developing small molecules or antibodies that either stabilize C1-inhibitor, enhance its activity, or, in specific contexts, transiently inhibit its function for research purposes. Each approach has its own advantages regarding specificity, delivery, and duration of action, and the choice depends on the therapeutic goal.
Q: What are the potential challenges in developing C1-inhibitor-based therapeutics?
A: Challenges can include ensuring adequate systemic exposure and tissue distribution, particularly for large protein therapeutics. Maintaining stability and activity in vivo can also be complex. Additionally, the intricate balance of the complement system means that modulating one component, like C1-inhibitor, requires careful consideration to avoid unintended consequences or paradoxical effects on other pathways.
Q: How can one assess the efficacy of a C1-inhibitor modulator in a preclinical setting?
A: Efficacy can be assessed through a combination of in vitro and in vivo assays. In vitro, this includes measuring the modulator's direct effect on C1-inhibitor activity, its binding affinity to target proteases, and its ability to regulate complement pathway activation in cell-based assays or human serum. In vivo, relevant animal models of complement-mediated diseases are used to evaluate the modulator's impact on disease progression, inflammation markers, and complement component levels.
Access the Creative Biolabs Edge – Request Your Quote Now
Related Hot Products
Featured Services
Reference
-
Panagiotou, Anneza, Marten Trendelenburg, and Michael Osthoff. "The lectin pathway of complement in myocardial ischemia/reperfusion injury—review of its significance and the potential impact of therapeutic interference by C1 esterase inhibitor." Frontiers in Immunology 9 (2018): 1151. DOI:10.3389/fimmu.2018.01151. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:


 Fig.2 Engagement of C1INH with plasma cascades.1
Fig.2 Engagement of C1INH with plasma cascades.1