Introduction of Complement Factor D
Complement Factor D (CFD), encoded by gene CFD in humans, is a serine protease that catalyzes the cleavage of factor B, the initial proteolytic step of the alternative pathway of complement activation. Factor D was originally identified as adipsin, a cell signaling protein secreted by adipocytes, myeloid cells, and hepatocytes, which regulates fat deposition and insulin secretion in mice. The molecular weight of factor D is about 25,000 daltons, composed of 253 amino acids to form a single chain with glycosylated modification.
Structure of Complement Factor D
Factor D is a serine protease that circulates as a mature protease and constitutively in the serum at low concentration. The structure of factor D is characterized by an atypical active site architecture comprising the catalytic triad in an inactive conformation, a self-inhibitory loop (aa 214-218) restricting access to the non-prime recognition sites, and an Arg218-Asp189 salt bridge at the bottom of the S1 pocket, which exhibits a highly restricted specificity and appears to be substrate activated. The protease does not recognize peptide substrates, but it still exerts weak catalytic activity against synthetic lysine-derived thioesters. The protein exhibits a pI=7.4.
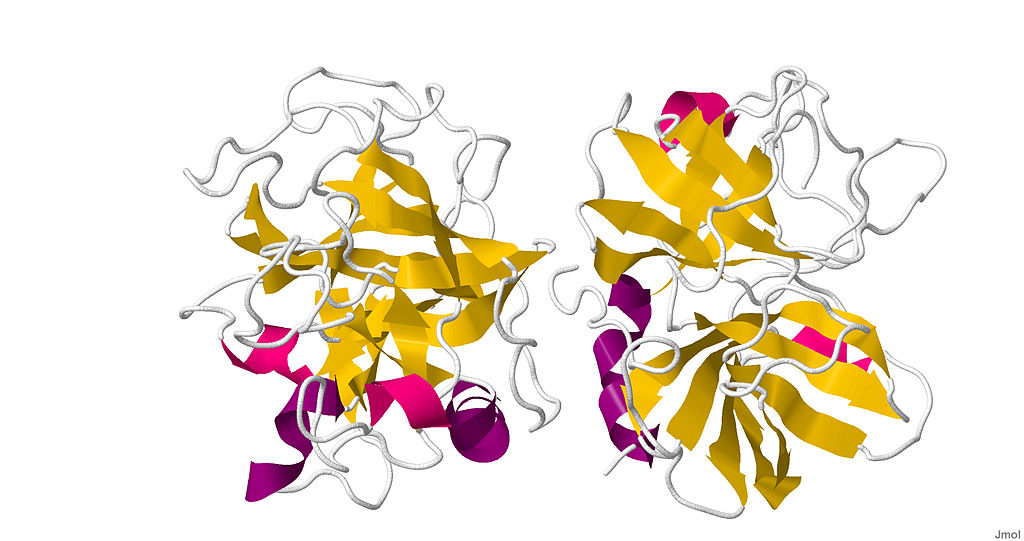 Distributed under CC0, from Wiki,
without modification.
Distributed under CC0, from Wiki,
without modification.
Fig.1 Structure of Factor D in Homo sapiens.
Function of Complement Factor D
As a trypsin-like S1 serine protease, factor D plays a key role in both alternative pathway (AP)-initiated C3 activation and subsequent amplification of this central activation step. Factor D only cleaves factor B when factor B is bound to C3b or a conformationally altered C3 such as C3(H2O) to generate an active C3 convertase (C3bBb), cleaving multiple C3 into C3b to generate more C3 convertase in a powerful amplification loop, resulting in the full activation of the complement system. C3b is deposited onto acceptor surfaces (opsonization). Recruitment of a further C3b molecular to the membrane-bound C3bBb complex generates a C5 convertase, leading to cleavage of C5 into C5a and C5b. C5b recruits C6, C7, C8 C9 to form membrane attack complex (MAC) on targeted surfaces with subsequent membrane disruption and cell lysis. Besides the important functions in complement system, factor D is demonstrated to participate in the stimulation of triglyceride synthesis in adipose tissue and acts as an inhibitor of neutrophil degranulation. Furthermore, lack of factor D predisposes to Neisseria infections.
Regulation and Applications of Complement Factor D
Because of its irreplaceable role in the activation of alternative pathway, factor D has attracted increasing attention on its regulation and applications. A lot of pathological damage is induced by primary or secondary activation of the alternative pathway, in which factor D is one of the most key elements. So many pharmaceutical companies have investigated various drugs as effective treatment for factor D associated diseases. Due to the distorted active site, except when bound to its substrate C3bBb, effective small molecule inhibitors have not yet been found. Meanwhile, none of the protease inhibitors in plasma affect factor D function.
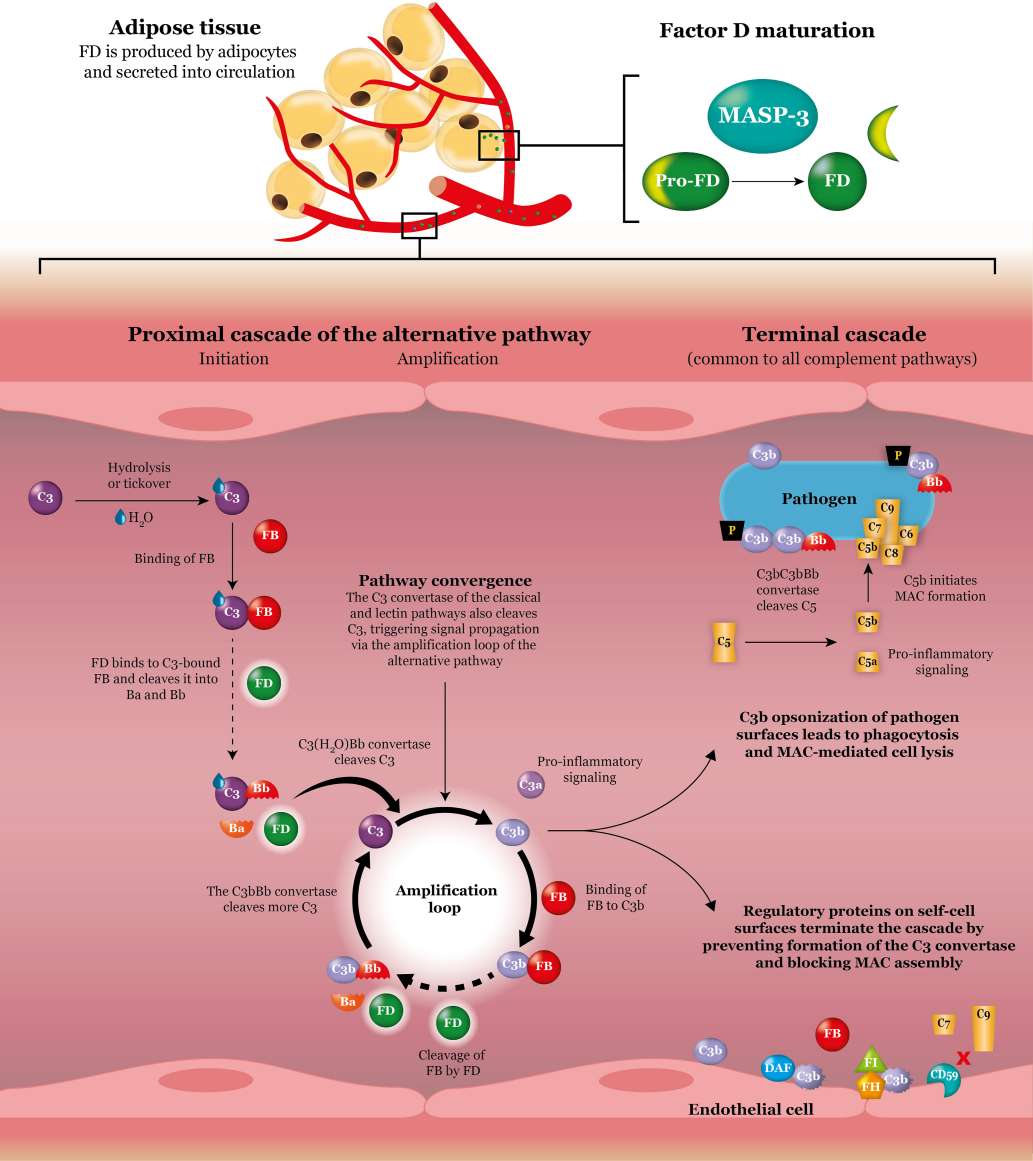
Fig.2 Factor D in the alternative complement pathway binds and cleaves factor B specifically.1, 3
With rich research experiences in drug discovery and complement therapeutic field, Creative Biolabs' outstanding research team are providing high-quality biotherapeutics development services based on the complement system. We offer turn-key or ala carte services customized to our client’s needs. If you are interested in our platform or you are calling for our services, please contact us for detailed information.
Published Data
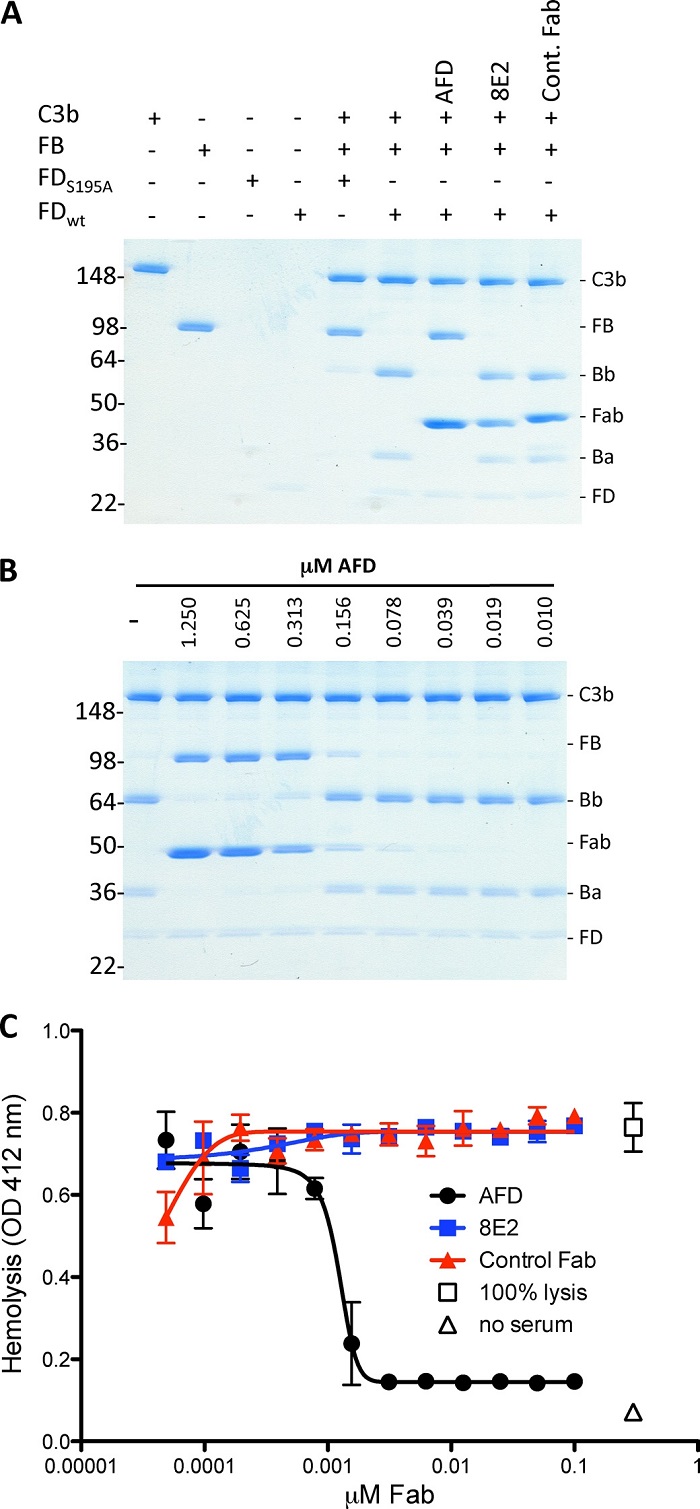 Fig.3 An anti-factor d Fab fragment blocks activation of C3bB proconvertase.2, 3
Fig.3 An anti-factor d Fab fragment blocks activation of C3bB proconvertase.2, 3
The alternative complement pathway, noted for its ability to enhance immune responses, is linked to several inflammatory conditions and presents an appealing target for therapy. An anti-factor D Fab fragment (AFD) has been developed to inhibit this pathway, specifically in advanced dry age-related macular degeneration. AFD effectively blocks the proteolytic activation of the macromolecular substrate C3bB by factor D, while not affecting the breakdown of a small synthetic substrate, indicating that AFD doesn't obstruct the substrate's access to the catalytic site. Structural analyses of AFD with human and cynomolgus factor D reveal that AFD binds to surface loops, part of the factor D exosite, thereby hindering macromolecular substrate access without affecting factor D's catalytic activity. This provides insight into the molecular mechanism underpinning AFD's inhibition of a crucial step in the alternative complement pathway.
References
-
Barratt, Jonathan, and Ilene Weitz. "Complement factor D as a strategic target for regulating the alternative complement pathway." Frontiers in immunology 12 (2021): 712572.
-
Katschke, Kenneth J., et al. "Inhibiting alternative pathway complement activation by targeting the factor D exosite." Journal of Biological Chemistry 287.16 (2012): 12886-12892.
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Product
Questions & Answer
A: While other complement-targeted therapies might focus on upstream components of the complement cascade, such as C3 or C5, complement factor D inhibitors specifically target the alternative pathway by blocking the cleavage of factor B. This targeted approach could offer a more precise and potentially safer way to modulate complement activation.
A: Researchers are exploring various strategies to target complement factor D, including monoclonal antibodies, small molecule inhibitors, and RNA interference (RNAi) approaches. Monoclonal antibodies can specifically bind to factor D, inhibiting its activity. Small molecule inhibitors can be designed to block the enzymatic function of factor D. RNAi-based therapies aim to silence the expression of factor D at the genetic level.
A: Several pharmaceutical companies and research groups are actively developing complement factor D inhibitors. Clinical trials are underway to assess the safety and efficacy of these inhibitors in treating various diseases. Early results have shown promise, but further research is needed to establish their therapeutic potential fully.
For Research Use Only.
Related Sections:



 Fig.3 An anti-factor d Fab fragment blocks activation of C3bB proconvertase.2, 3
Fig.3 An anti-factor d Fab fragment blocks activation of C3bB proconvertase.2, 3