Serpins are known for their unusual mechanism of action. Unlike the common competitive mechanism of protease inhibitors that bind to protease active sites and block their entry, serpins irreversibly inhibit the function of their protease by undergoing large conformational changes to disrupt the active site of it. For years, Creative Biolabs has consistently and effectively supported the anti-complement therapy industry with a unique range of products and related services. We provide biotherapeutics development strategies for serine protease inhibitors including serpins, macroglobulins, and other small protein inhibitors.
Structure of Serpins
Serpins form a large group of homologous proteins which appear to be ubiquitous in multicellular eukaryotes. They consist of about 400 amino acids and are often glycosylated. Serpins fold into an NH2-terminal helix domain and a COOH-terminal beta-sheet domain. A serpin protease folding protein consisting of 3 beta-sheets (A-C) and 8 or 9 alpha helix linkers and an exposed 20-residue RCL is used as bait for the protease target.
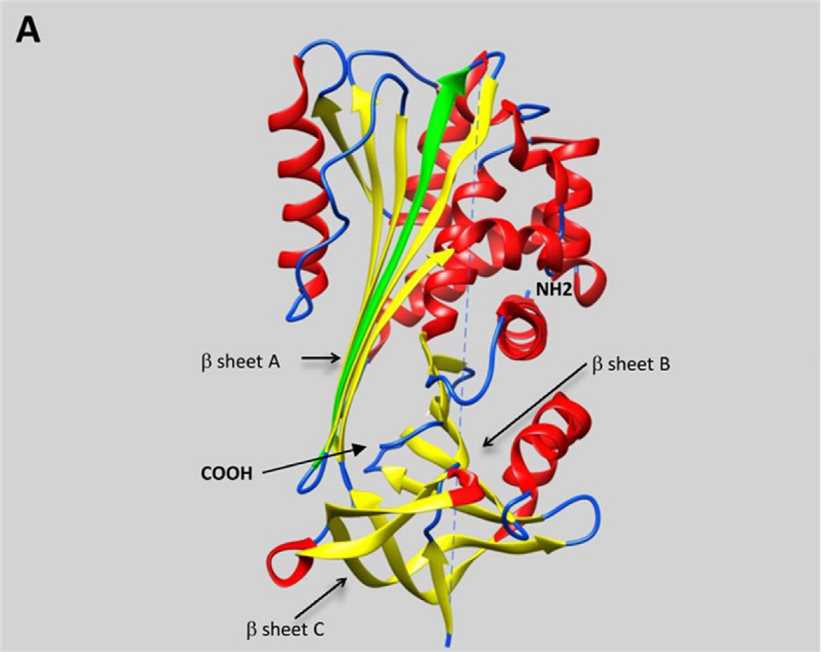
Fig. 1 Representative schematic diagram of secondary structures of serpins.1, 2
Biological Function and Inhibitory Mechanism
An unusual aspect of serpins is their native unstable fold, with the RCL on the top and the beta-sheet A on the outside. After proteolysis, the RCL is cleaved and the amino terminal portion of the RCL is inserted into the center of the beta-sheet A to form an additional (fourth) strand (s4A) which can effectively stabilize the complex structure. The protease is therefore denatured, and the serine protease inhibitor/protease complex is degraded. This amino-terminal RCL insertion can occur proteolytically when cleaved or spontaneously. As a result, serine protease inhibitors interact with their targets through "suicide- cleavage" mechanisms, leading to the formation of inactive serpin/protease complexes.
Serpins Related Diseases
Serine protease inhibitor is one of the targets of medical research because it involves a series of biological processes, including coagulation and inflammation. Their unique conformational changes have also sparked interest in the structural biology and protein folding research communities. The mechanism of conformational change has advantages but also disadvantages: serpins are vulnerable to mutations that can lead to serpinopathies, such as protein misfolding and the formation of inactive long chain polymers. Mutations that alter serine protease activity, specificity, or aggregation properties all affect their function. Most diseases associated with serpins are the result of serpin polymerization into aggregates. Serpin polymerization not only reduces the amount of active inhibitor but also causes polymer accumulation, which leads to cell death and organ failure.
Therapeutic Strategies
Several treatments are being used or are currently under investigation to treat most common serpinopathy: antitrypsin deficiency. Antitrypsin-boosting therapy is approved for the treatment of pulmonary emphysema with severe antitrypsin deficiency. In animal models, gene targeting in induced pluripotent stem cells has been successfully used to correct defects in antitrypsin polymerization and restore the mammalian liver's ability to secrete active antitrypsin. Small molecules have also been developed to block the antitrypsin polymerization in vitro. In clinical therapy, antiserum is purified from plasma of blood donors and administered intravenously. Lung and liver transplants have been proven effective for the treatment of severe conditions related to antitrypsin deficiency.
As one of the most highly respected drug discovery and antibody development laboratories in the world, Creative Biolabs has been helping customers all over the world make fast and effective biotherapeutics development decisions for decades. We provide biotherapeutics development strategies for serine protease inhibitors including serpins, macroglobulins, and other small protein inhibitors. Our scientists, technicians and regulatory experts are committed to assisting your research with our complement-based R&D services. Please contact us for more information and a detailed quote.
References
-
Blisnick, Adrien A., Thierry Foulon, and Sarah I. Bonnet. "Serine protease inhibitors in ticks: an overview of their role in tick biology and tick-borne pathogen transmission." Frontiers in Cellular and Infection Microbiology 7 (2017): 199.
-
under Open Access license CC BY 4.0, The image was modified by extracting and using only Part A of the original image.
Related Product
For Research Use Only.
Related Sections:

