Proteases play important roles in human physiology and pathology. Complement systems are proteolytic cascades in which serine proteases are activated from each other by limited proteolysis in a strictly ordered manner. Since uncontrolled complement activation contributes to the development of serious diseases, inhibition of the complement serine proteases may be an attractive therapeutic approach. For years, Creative Biolabs has consistently and effectively supported the anti-complement therapy industry with a unique range of products and related services. We provide biotherapeutics development strategies based on serine protease inhibitors including serpins, macroglobulins, and other small protein inhibitors.
There are two types of natural serine protease inhibitors. The first type undergoes major conformational change during the inhibitory process and form irreversible complexes with the target proteases. These two classes of inhibitors are serpins and macroglobulins, which are large protein inhibitors and are abundant in the blood. The second type, small protein inhibitors of serine proteases can form reversible complexes with the target protease by thermodynamic terms. Although no covalent bonds are formed between the two proteins, the binding affinity can be so high that the interaction is actually irreversible.
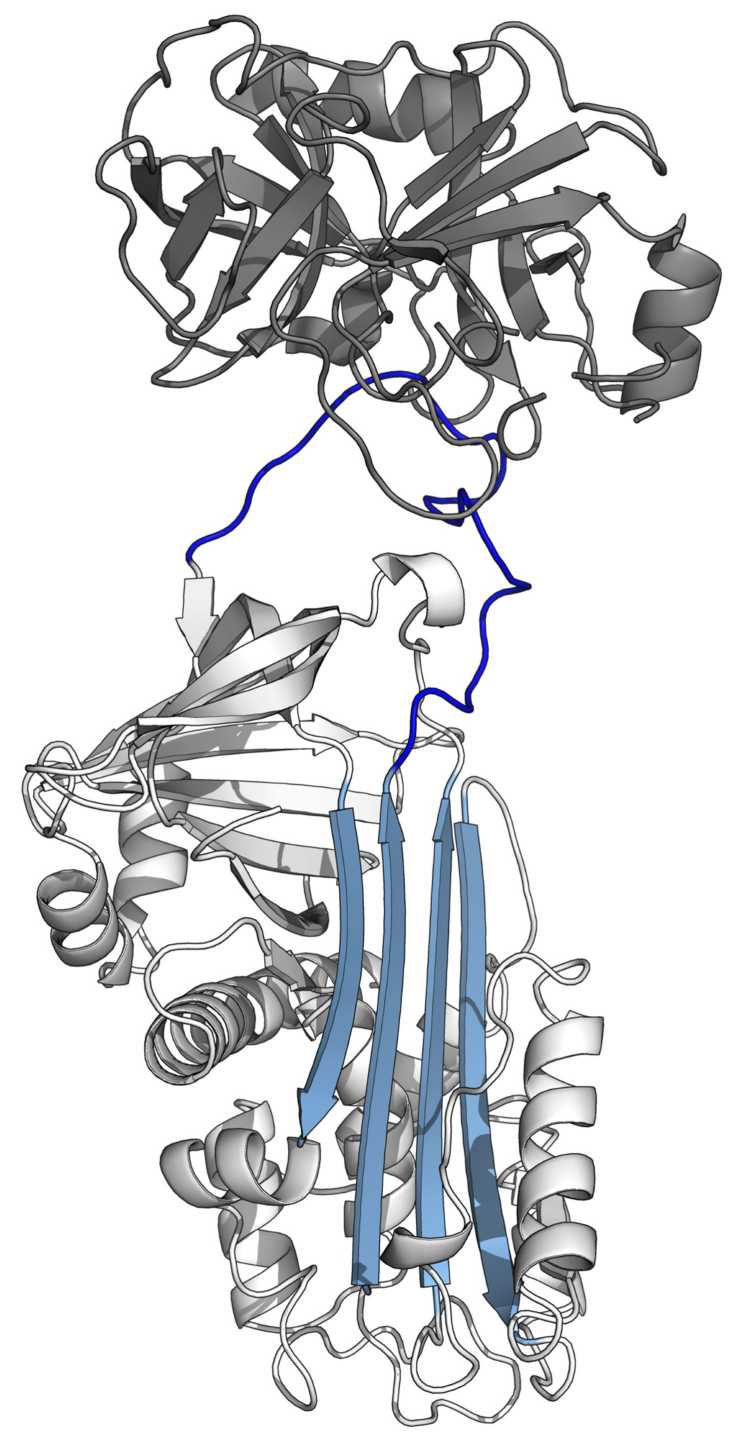
Fig. 1 The structure of serine protease inhibitor.1
Serpins
Serpins are known for their unusual mechanism of action. Unlike the common competitive mechanism of protease inhibitors that bind to protease active sites and block their entry, serpins irreversibly inhibit the function of their protease by undergoing large conformational changes to disrupt the active site of it. Serpins are vulnerable to mutations that can lead to serpinopathies, such as protein misfolding and the formation of inactive long chain polymers. Mutations that alter serine protease activity, specificity, or aggregation properties all affect their function. Most diseases associated with serpins are the result of serpin polymerization into aggregates. Serpin polymerization not only reduces the amount of active inhibitor but also causes polymer accumulation, which leads to cell death and organ failure.
Macroglobulins
α2-Macroglobulin (α2M) is the largest major non-immunoglobulin protein in human plasma. Members of the α2M group have been identified in a wide range of vertebrate and invertebrate species and comprise the C3, C4, and C5 components of the vertebrate complement system. α2Ms are thought to be early innate immune components, similar to opsonin, but their role in proteolytic attack of invading pathogens remains hypothetical. alpha-2-Macroglobulins (α2-Ms) act as an anti-protease, inhibit fibrinolysis by inhibiting plasmin and kallikrein and therefore inactivate an enormous variety of proteinases.
Small Protein Inhibitors
Small protein inhibitors of serine proteases can form reversible complexes with the target protease by thermodynamic terms. Although no covalent bonds are formed between the two proteins, the binding affinity can be so high that the interaction is actually irreversible. These tight-binding inhibitors fall into two broad categories: the canonical and noncanonical inhibitors. The canonical and noncanonical inhibitors both block the active site of the inhibited enzyme. The canonical inhibitors are typically small (shorter than 100 amino acids) proteins or domains of mosaic protein with different scaffolds but with exposed inhibitory loop that always show the same (canonical) main-chain conformation. Such inhibitors follow the standard Laskowski mechanism. This tight lock and key protein-protein interactions involve antiparallel beta-sheet. The unconventional inhibitors cannot mimic the substrate. The N-terminal segment of the inhibitor binds to the active site of the protease, forming a short parallel beta-sheet. The most striking feature of these inhibitors is that they form extra extensive secondary interactions outside the active site, which results in a very high affinity.
As one of the most highly respected drug discovery and antibody development laboratories in the world, Creative Biolabs has been helping customers all over the world make fast and effective biotherapeutics development decisions for decades. We provide biotherapeutics development strategies for serine protease inhibitors including serpins, macroglobulins, and other small protein inhibitors. Our scientists, technicians and regulatory experts are committed to assisting your research with our complement-based R&D services. Please contact us for more information and a detailed quote.
Reference
1. From Wikipedia: By Thomas Shafee - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/wiki/File:Serpin_(stressed).png
Related Product
For Research Use Only.
Related Sections:

