Alpha-2-Macroglobulins (α2-Ms) act as an anti-protease, inhibit fibrinolysis by inhibiting plasmin and kallikrein and therefore inactivate an enormous variety of proteinases. For years, Creative Biolabs has consistently and effectively supported the anti-complement therapy industry with a unique range of products and related services. We provide biotherapeutics development strategies for serine protease inhibitors including Serpins, Macroglobulins, and other Small Protein Inhibitors.
Structure of Alpha-2-Macroglobulins
α2-Macroglobulin (α2M) is the largest major non-immunoglobulin protein in human plasma. Members of the α2M group have been identified in a wide range of vertebrate and invertebrate species and comprise the C3, C4, and C5 components of the vertebrate complement system. α2Ms are thought to be early innate immune components, similar to opsonin, but their role in the proteolytic attack of invading pathogens remains hypothetical. Most α2Ms are tetramers assembled from paired subunits with disulfide bridges, but there are also monomeric and dimeric forms, the latter being more common in invertebrates.
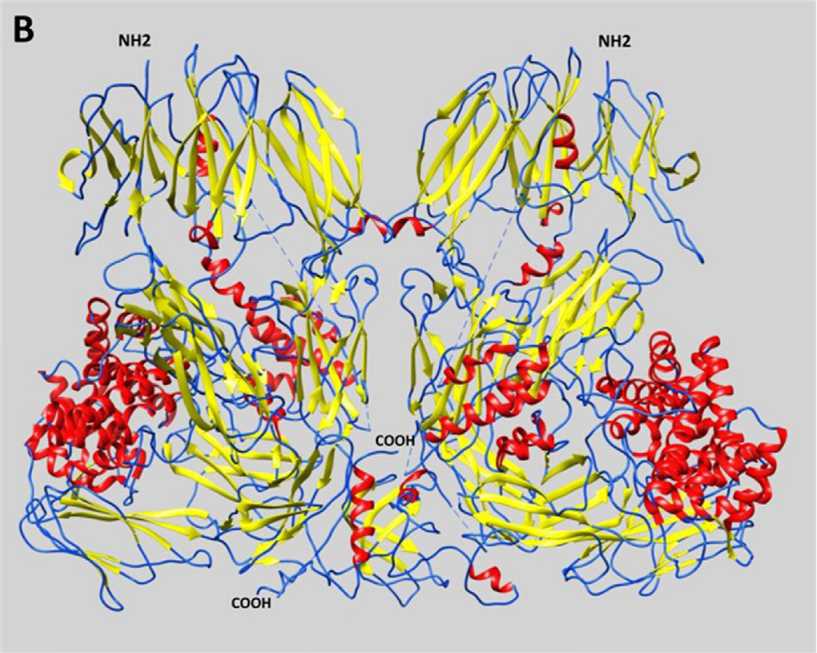
Fig. 1 Representative schematic diagram of secondary structures of α2-Macroglobulins.1
Biological Function and Inhibitory Mechanism
The α-macroglobulin protein family includes protease inhibitors, typified by human tetramers alpha-2-macroglobulin. They belong to the MEROPS proteinase inhibitor family I39, clan IL, which share several defining properties including a similar protease inhibitory mechanism and inhibiting proteases from all catalytic classes. α2M is capable of inactivating a large number of proteases including aspartate-, cysteine-, serine-, and metalloproteinases). It acts as a fibrinolytic inhibitor by inhibiting plasmin and kallikrein and as a coagulation inhibitor by inhibiting thrombin. α2M has a 35-amino acid “bait region” in its structure and proteinases binding and cleaving the bait region become bound to α2M. The protease-α2M complex is recognized by macrophage receptors and cleared from the system.
α2M Related Diseases
-
Nephrotic syndrome: a condition where the kidneys begin to leak some of the smaller blood proteins. When serum albumin levels are low, α2M levels are elevated, which is most commonly seen in nephrotic syndrome. α2M is retained in the blood due to its size. Increased production of all proteins means increased α2M concentrations. This increase has little effect on health but can be used as a diagnostic clue. Long-term chronic renal failure can lead to amyloid by α2M.
-
Alzheimer's disease (AD): α2M is also produced in the brain. It binds to multiple extracellular ligands and is internalized by neurons and astrocytes. In the brains of patients with Alzheimer's disease, α2M has been localized to diffuse amyloid plaques. α2M also binds to soluble beta-amyloid, of which it mediates degradation. However, an excess of α2M may also have a neurotoxic effect. Based on genetic evidence, it is now confirmed as one of two confirmed late-onset AD genes.
As one of the most highly respected drug discovery and antibody development laboratories in the world, Creative Biolabs has been helping customers all over the world make fast and effective biotherapeutics development decisions for decades. We provide biotherapeutics development strategies for serpins. Our scientists, technicians and regulatory experts are committed to assisting your research with our complement-based R&D services. Please contact us for more information and a detailed quote.
References
-
Blisnick, Adrien A., Thierry Foulon, and Sarah I. Bonnet. "Serine protease inhibitors in ticks: an overview of their role in tick biology and tick-borne pathogen transmission." Frontiers in Cellular and Infection Microbiology 7 (2017): 199.
-
under Open Access license CC BY 4.0, The image was modified by extracting and using only Part B of the original image.
Related Product
For Research Use Only.
Related Sections:

