ADA Confirmatory Assays
Focusing on the industry of preclinical drug discovery for many years, Creative Biolabs is able to provide a comprehensive series of ADA immunogenicity assessment services for global customers. Particularly, we offer ADA confirmatory assays to exclude any potential false-positive results obtained from ADA screening assays.
Introduction of ADA Confirmatory Assays
ADA immunogenicity evaluation is typically evaluated using both screening and confirmatory assay methods in a tiered manner. In the first tier, ADA screening assays are used to detect all antibodies that bind to drug. The second tier namely ADA confirmatory assays are conducted to confirm the results of screening assays. In other words, samples testing positive in the screening assay are then subjected to a confirmatory assay to demonstrate that ADA is specific for the therapeutic protein product. For example, a competition assay could confirm that antibody is specifically binding to the therapeutic protein product and that the positive finding in the screening assay is not a result of non-specific interactions of the test serum or detection reagent with other materials in the assay milieu such as plastic or other proteins.
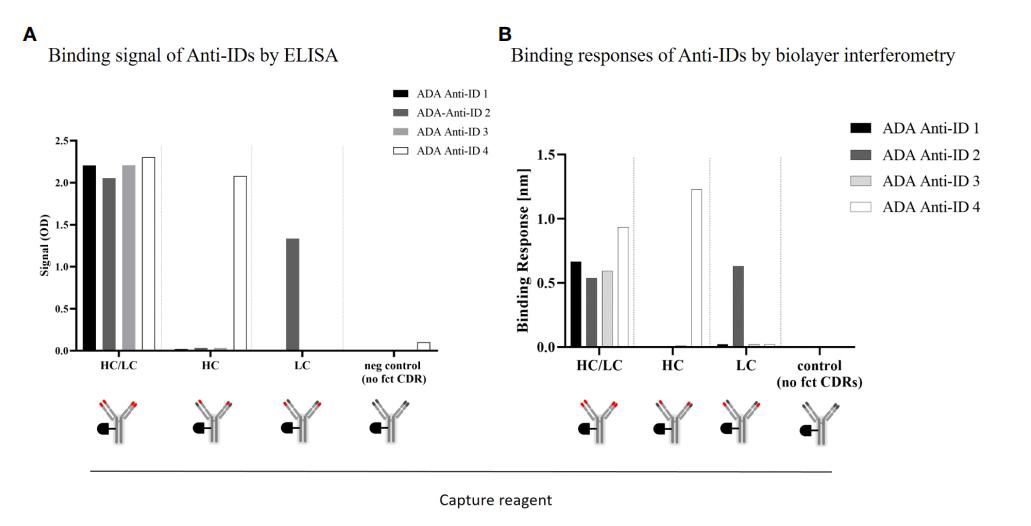 Fig.1 ADA anti-CD3/CDR domain specificity characterization: Binding characteristics of four different monoclonal anti-idiotypic antibodies to modified anti-CD3 domains analyzed by ELISA.1, 2
Fig.1 ADA anti-CD3/CDR domain specificity characterization: Binding characteristics of four different monoclonal anti-idiotypic antibodies to modified anti-CD3 domains analyzed by ELISA.1, 2
ADA Confirmatory Assays Provided by Creative Biolabs
As the ADA screening assay is designed to broadly detect the presence of antibodies that bind biological drug in serum samples with a defined false-positive rate, it is recommended that there is a need to develop assays to confirm the binding of antibodies that are specific to the therapeutic protein product. Implementation of a suitable confirmatory assay is important to prevent data on ADA false-positive patients from confounding the analyses of the impact of ADA on safety and efficacy. Generally, the method selected may be similar to that used in the screening assay. With years of experience, Creative Biolabs is capable of helping you select the most suitable format to determine the specificity between therapeutic protein product and anti-drug antibody, including but not limited to:
- Direct/Indirect ELISA
- Bridging ELISA
- Electrochemiluminescence (ECL)
- Radioimmunoprecipitation assay (RIPA)
- Surface plasmon resonance (SPR)
Features of our Services
- Comprehensive: Full-scale ADA confirmatory assays with high sensitive
- Cost-effective: Competitive price with the best quality
- Quickly: Short turnaround time with advanced technologies
- Professionally: Assisted by professional technical team
Having worked in the field of immunogenicity assessment for many years, Creative Biolabs has successfully provided a series of high-quality services for worldwide customers. Except for ADA confirmatory assays, we also offer other immunogenicity assessment services. To learn more information, please feel free to contact us.
References
- Lotz, Gregor P., et al. "Characterization of anti-drug antibody responses to the T-cell engaging bispecific antibody cibisatamab to understand the impact on exposure." Frontiers in Immunology 15 (2024): 1406353.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
