Web based Immunogenicity Screening
Creative Biolabs has established an incomparable web-based immunogenicity screening platform. With our experienced scientists, our team can provide high-quality immunogenicity analysis services to help global customers find potential factors that lead to immunogenicity.
Introduction of Web-based Immunogenicity Screening
During the past years, the unwanted immunogenicity of biological drugs has greatly hampered the development of new therapies into the market, as it possibly compromises drug safety and alters pharmacokinetics. Studies have shown that the immunogenicity of therapeutic protein is driven by both external and intrinsic factors. Among the external factors are dosage, frequency, mode of administration, purity, formulation of the product, genetic background, concomitant use of immunosuppressive drugs, and the disease state of the patient. On the other hand, B and T cell epitopes are an intrinsic part of the therapeutic protein and their presence is essential for the development of antibody responses. Web-based immunogenicity screening is an in silico method that was established to identify potential cell epitopes of virtually all human leukocyte antigen molecules from a wide genetic background.
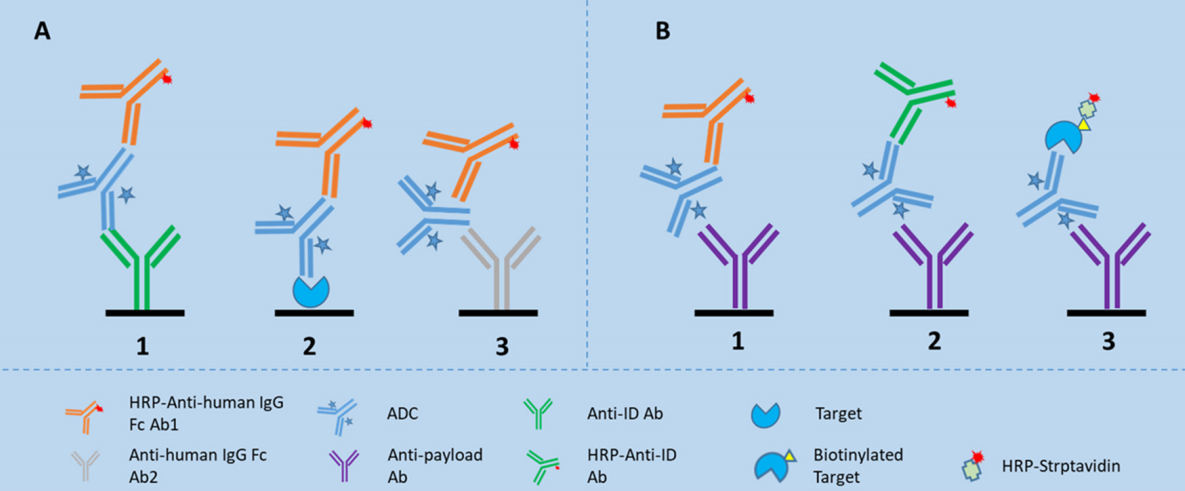 Fig.1 Bridging assay format for total ADA detection and two assay formats, namely epitope competition method and epitope detection method for domain specificity testing.1, 2
Fig.1 Bridging assay format for total ADA detection and two assay formats, namely epitope competition method and epitope detection method for domain specificity testing.1, 2
Web-based Immunogenicity Screening Services Provided by Creative Biolabs
With advanced technology and professional scientists, Creative Biolabs has successfully established a web-based immunogenicity screening platform for high-throughput screening of partial and complete sequences of biological drugs even in the very early development cycle, such as lead selection. Based on the cutting-edge technology platform, we can help our clients identify potentially immunogenic regions within each protein sequence. Especially, the services we can provide include:
- Screen the protein/antibody sequences of drug candidates and identify potential T-cell and B-cell epitope clusters contained within product candidates
- Map epitope through a combination of predictive conformational modeling and linear interaction mapping
- Rate the potential immunogenic of each epitope cluster and compare each epitope cluster to other well-known immunogenic epitope clusters
Features of Our Services
- Fast turnaround time and low cost
- Customized service to best meet the needs and preferences of your biologics team
- Professional technical support team
- High-quality after-sale service
Aided by advanced web-based immunogenicity screening platform, Creative Biolabs is able to help worldwide customers identify cell epitopes from a wide genetic background. The services we provide will be presented in a relatively inexpensive manner. In addition to web-based immunogenicity screening service, we also provide other immunogenicity assessment services for global customers. We are confident to promote your project a success. If you are interested in the services we provide, please feel free to contact us for more information.
References
- Qin, Qiuping, and Likun Gong. "Current Analytical Strategies for Antibody–Drug Conjugates in Biomatrices." Molecules 27.19 (2022): 6299.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
