Antibody Immunogenicity Assessment Services
Detection and analysis of anti-drug antibody (ADA) formation are vital in understanding potential immune responses caused by therapeutic protein products which greatly affect the design of preclinical programs for biotechnology products. As an internationally active Contract Research Organization (CRO), Creative Biolabs provides comprehensive antibody immunogenicity assessment services to promote therapeutic protein product development. With experienced scientists, our immunoanalytics department can help you select the most suitable assay to measure ADA responses of your biologic.
Importance of Immunogenicity Assessment
Immunogenicity is a significant concern for therapeutic protein products in preclinical development as it has the potential to affect product pharmacokinetics, pharmacodynamics, safety, and efficacy. The consequences of immunogenicity are highly variable, ranging from no evidence of clinical effect to severe allergic or anaphylactic reactions, reduction in efficacy, or induction of autoimmunity. Detection and analysis of ADA formation is a helpful tool in understanding potential immunogenicity. Therefore, the development of valid, sensitive, specific, and selective assays to measure ADA responses is essential for biological drug development. Generally, a panel of assays are required to provide necessary information on the immunogenicity profile of a biotherapeutic.
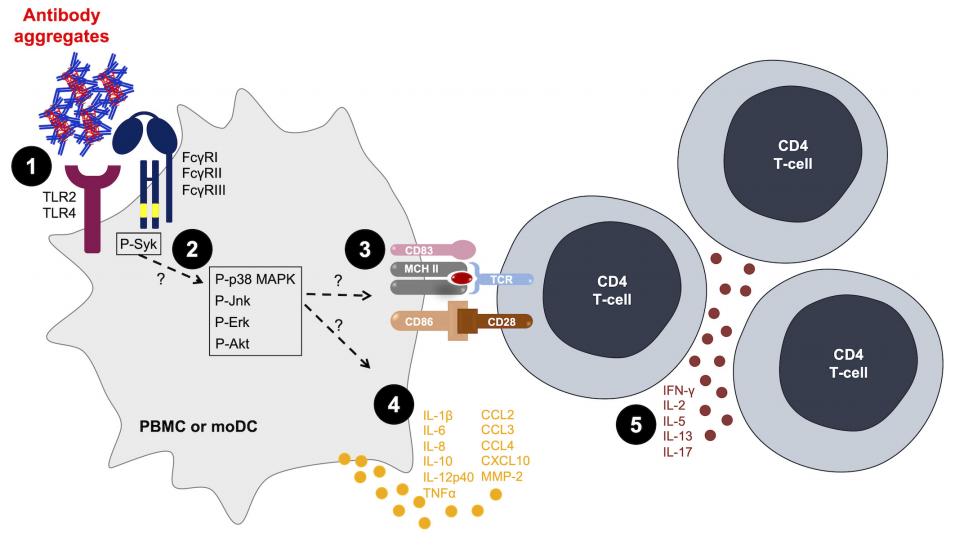 Fig.1 Antibody aggregates act as danger signals for innate immune cells.1, 2
Fig.1 Antibody aggregates act as danger signals for innate immune cells.1, 2
ADA Screening Assays
ADA screening is a highly sensitive screening assay used to detect potential low- and high-affinity ADA in patient samples or samples from animals in the preclinical stage. Various methods are well-established for ADA screening, including ELISA, multi-titer assay, Electrochemiluminescence (ECL). Theoretically, a low but defined false-positive rate of approximately 5% is desirable for the initial screening assay because it maximizes the detection of true positives. Subsequent assays can be employed to exclude false-positive results when determining the true incidence of immunogenicity.
ADA Confirmatory Assays
ADA confirmatory assays aim to eliminate false-positive samples identified during the initial screening assay. For example, a confirmatory assay could confirm that whether an antibody is specifically binding to the therapeutic protein product and that the positive finding in the screening assay is not a result of non-specific interactions of the test serum or detection reagent with other materials in the assay milieu such as plastic or other proteins. Frequently, the method and instrument platform selected may be similar to those used for the screening assay.
ADA Characterization Assays
In addition to ADA screening and confirmatory assays, further ADA characterization assays should also be conducted to provide additional information for the understanding of a particular immune response. Generally, ADA characterization assay consists of two different assays, including titering testing and isotype analysis. Titering assays are used to characterize the magnitude of the ADA response. Whereas, the goal of isotype assays is to detect all relevant immunoglobulin (Ig) isotypes.
ADA Quantification Assays
ADA quantification aims to accurately detect ADA. Drug-tolerant assays have been developed to measure ADA in complex (using drug/ADA complex precipitation in combination with acidification). What's more, a series of methods have been exploited for ADA quantification, including but not limited to chemo-luminescence, radio-immune assay, and bridging ELISA.
Web-based Immunogenicity Screening
Web-based immunogenicity screening is an in silico method that was established to identify potential cell epitopes of virtually all human leukocyte antigen molecules from a wide genetic background. Based on this advanced technology, we can help our clients identify potentially immunogenic regions within each protein sequence and map individual amino acids which contribute to the immunogenic potential of the cluster.
Evaluation of Neutralizing Activity
Determination of the neutralizing potential of the induced antibodies is an essential element of immunogenicity evaluation as neutralizing antibodies (NAb) can interfere with the clinical activity of a therapeutic protein product by preventing the product from reaching its target or by interfering with its pharmacologic activity such as receptor-ligand interactions.
Pharmacokinetic / Toxicokinetic (PK/TK) Analysis
Studies have shown that the formation of ADA could alter the clearance rate, and interfere in the PK assay used to measure the drug concentration in circulation. Further, ADA can significantly confound the quantitation of drug levels in pharmacokinetic/toxicokinetic (PK/TK) analysis and may result in insufficient exposure to calculate safety margins from toxicology studies. In this case, the analysis of PK/TK changes caused by ADA is a critical part of preclinical drug development.
Highlights
Having worked in the field for many years, Creative Biolabs has gained a wealth of experience in antibody immunogenicity assessment. Most importantly, we have the expertise and resources to develop and validate methods as well as perform sample analysis to help you fully study the immunogenicity of your novel compounds in both discovery and preclinical stages. The services we offered are characterized as:
- One-stop service with both standard and customized protocols
- Flexible options
- Professional technical support team
- High-quality after-sale service
During the past years, Creative Biolabs has gained a good reputation for successfully offering reliable ADA immunogenicity evaluation services for global customers. We are committed to accelerating the development of your therapeutic protein drug development. If you are interested in our services, please don't hesitate to contact us for more information.
References
- Nabhan, Myriam, Marc Pallardy, and Isabelle Turbica. "Immunogenicity of bioproducts: cellular models to evaluate the impact of therapeutic antibody aggregates." Frontiers in immunology 11 (2020): 725.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
