ADA Characterization Assays
With years of experience in ADA immunogenicity evaluation, Creative Biolabs can offer ADA characterization services for our worldwide clients, to make it more timely and cost-effective for preclinical studies. Our team of technical experts will spare no effort in providing professional technical support.
Introduction of ADA Characterization Assays
Immunogenicity of biological products is a major concern in both pre-clinical and clinical studies. Besides screening and confirmatory assays, further ADA characterization assays should be conducted to provide additional information that is important for the understanding of a particular immune response. Generally, ADA characterization assay consists of two different assays, namely titering testing, and isotype analysis. Titering assays are used to characterize the magnitude of the ADA response. It is important to characterize this magnitude with titering assays because the impact of ADA on safety and efficacy may correlate with ADA titer and persistence rather than incidence. Whereas, the goal of isotype assays is to detect all relevant immunoglobulin (Ig) isotypes.
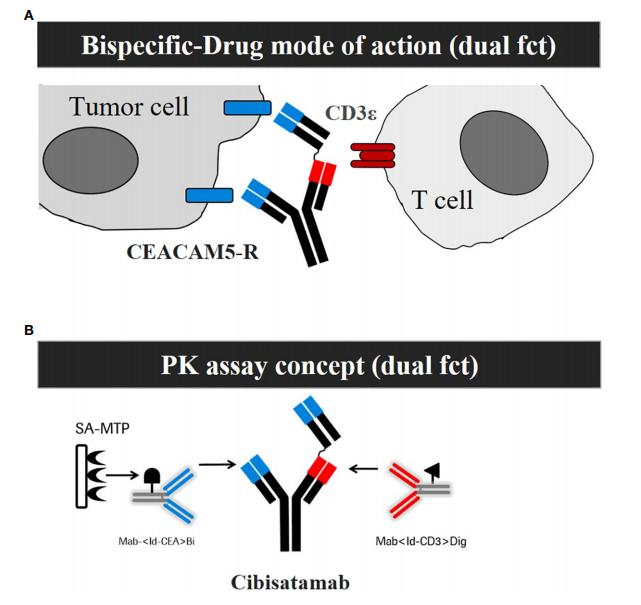 Fig.1 Development of a mechanism of action (MoA) based Pharmacokinetic (PK) assay that is sensitive for ADA impact on exposure.1, 2
Fig.1 Development of a mechanism of action (MoA) based Pharmacokinetic (PK) assay that is sensitive for ADA impact on exposure.1, 2
ADA Characterization Assays Available at Creative Biolabs
If a robust immune response occurs, characterization of the timing, strength, type, and specificity of the response provides a means to differentiate the nature of the ADA that can impact efficacy or safety. Therefore, it is a key part to determine all relevant immunoglobulin isotypes and functional titer in ADA characterization assays. For non-mucosal routes of administration, and in the absence of anaphylaxis, the expected ADA isotypes are IgM and IgG. For mucosal routes of administration, IgA isotype ADA is also expected. Aided by abundant experience and advanced technology, Creative Biolabs is able to provide both isotyping and titration testing services for global customers. We are committed to helping our clients with the following aspects.
- Multiple isotype determination (IgM, IgA, IgE, IgG, IgD)
- Determination of relative binding affinity
- Relative antibody concentration
Features of our Services
- Numerous approaches and flexible options
- Experienced technical team
- High-quality results with best after-sale service
- One-stop pipeline
Creative Biolabs is committed to being your good partner in new drug discovery development. With state-of-the-art technology, our professional staffs will offer you trustworthy expert solutions to meet your unique bioanalytical needs. And our scientists will help you select the most suitable experiment format for your biological products. In addition to ADA characterization assays, we also provide other ADA immunogenicity assessment services to meet every client's requirements. For more details and information, please feel free to contact us.
References
- Lotz, Gregor P., et al. "Characterization of anti-drug antibody responses to the T-cell engaging bispecific antibody cibisatamab to understand the impact on exposure." Frontiers in Immunology 15 (2024): 1406353.
- Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
